Korean J Physiol Pharmacol.
2010 Apr;14(2):91-97. 10.4196/kjpp.2010.14.2.91.
Pyrithione-zinc Prevents UVB-induced Epidermal Hyperplasia by Inducing HIF-1alpha
- Affiliations
-
- 1Department of Pharmacology, Chungbuk National University College of Medicine, Cheongju 361-763, Korea.
- 2Department of Molecular Cell Biology, Sungkyunkwan University School of Medicine, Suwon 440-746, Korea. khoonlee@skku.edu
- 3Department of Pharmacology, Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul 110-799, Korea. parkjw@snu.ac.kr
- KMID: 1457674
- DOI: http://doi.org/10.4196/kjpp.2010.14.2.91
Abstract
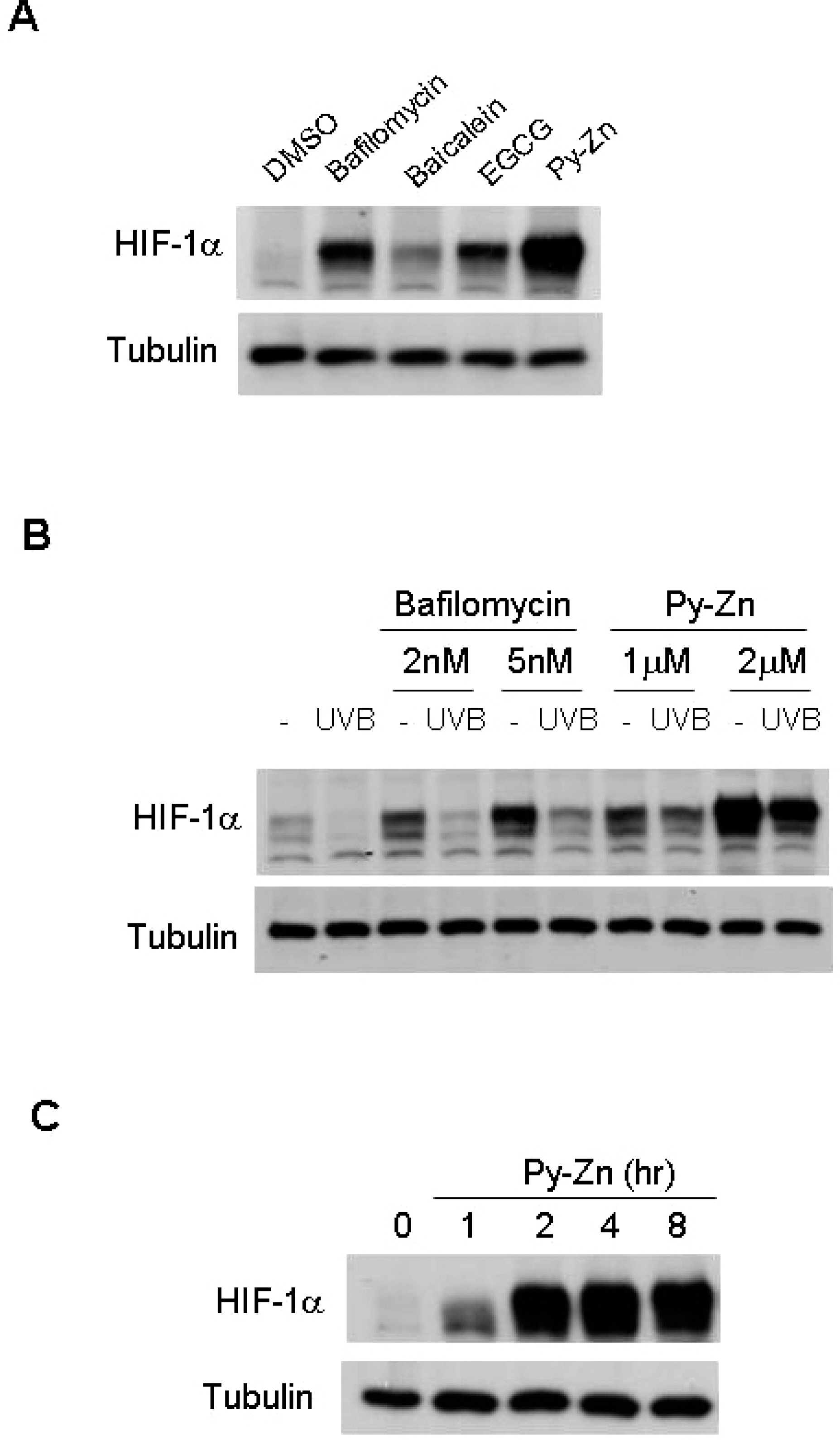
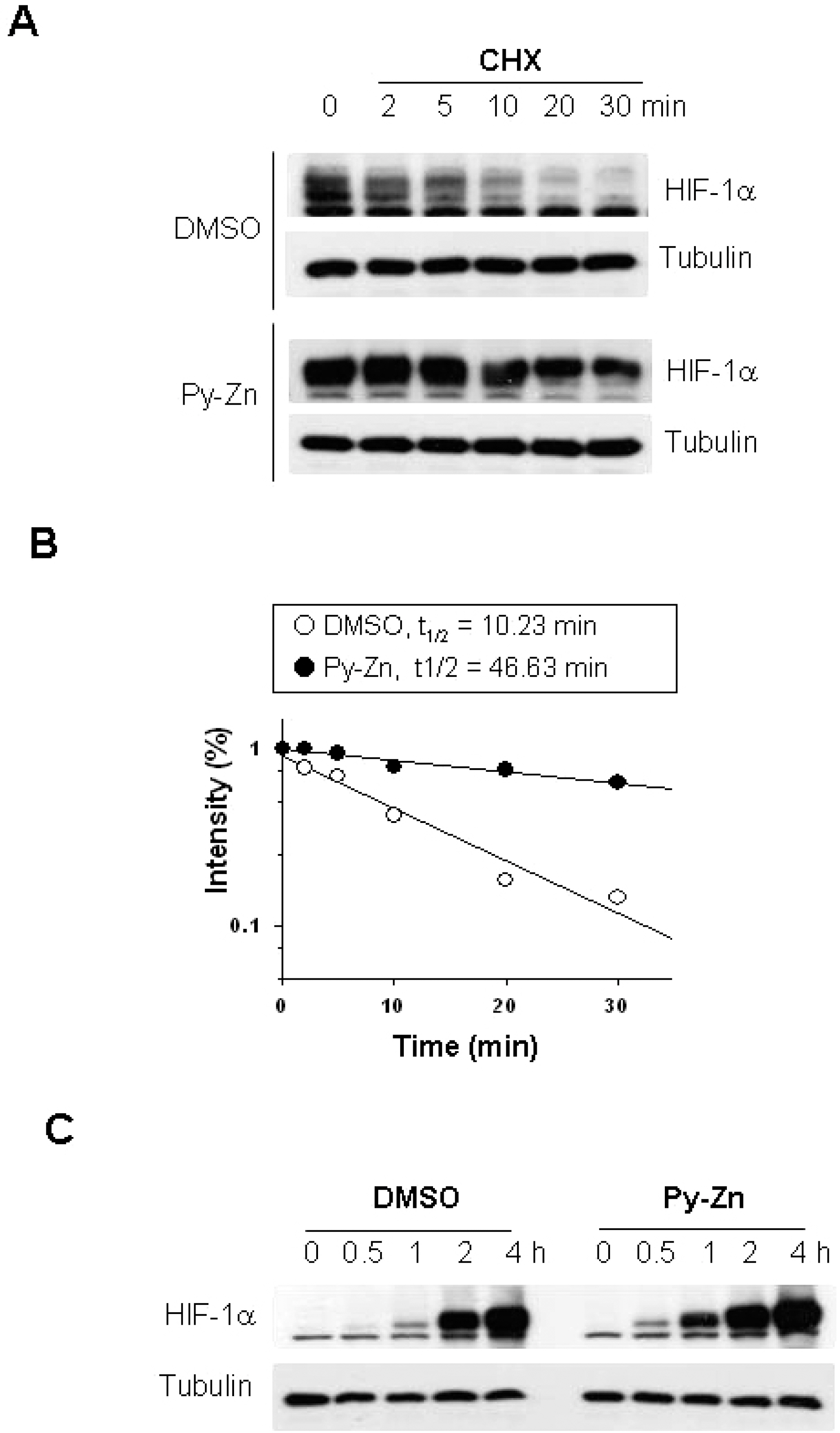
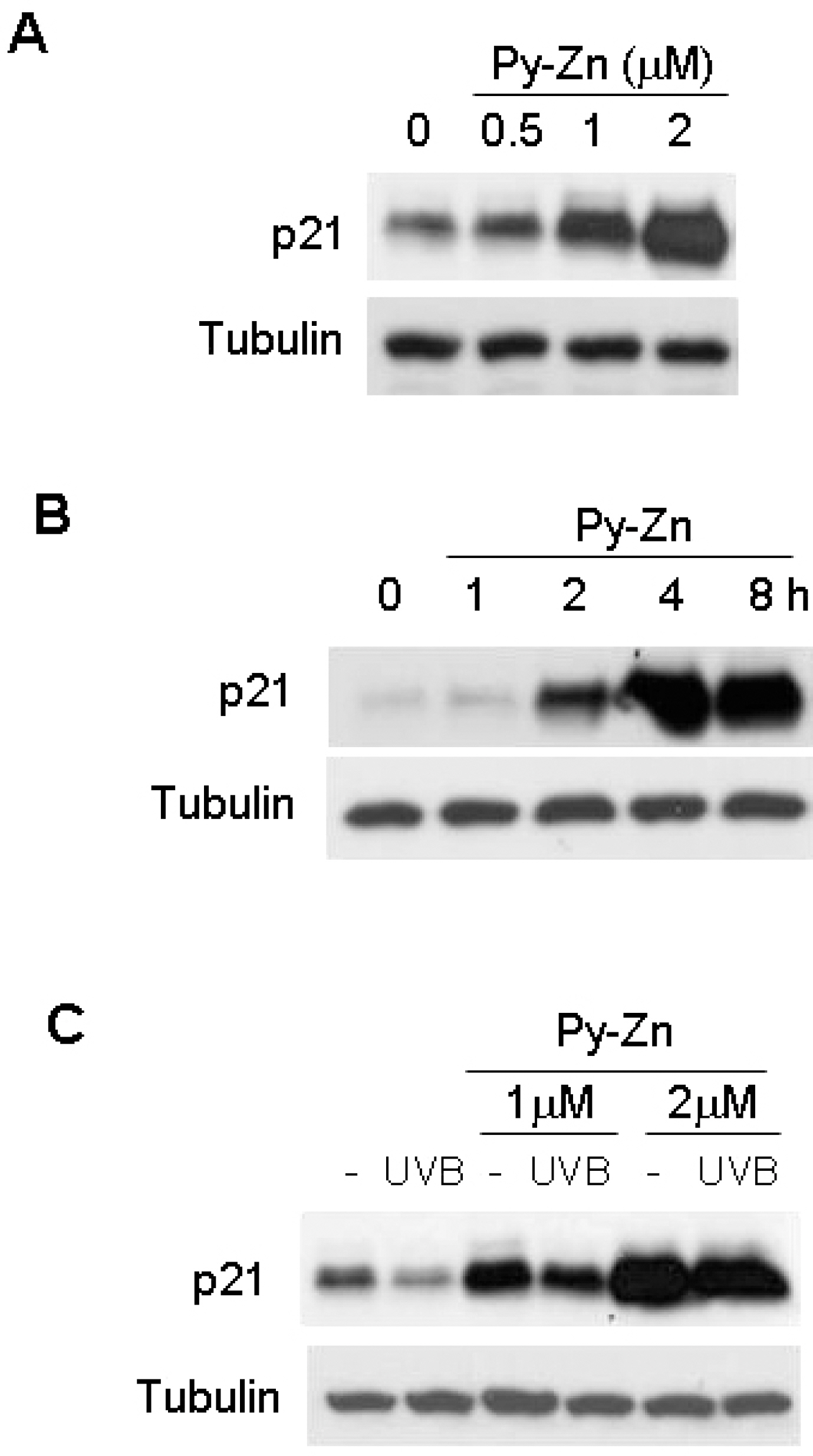
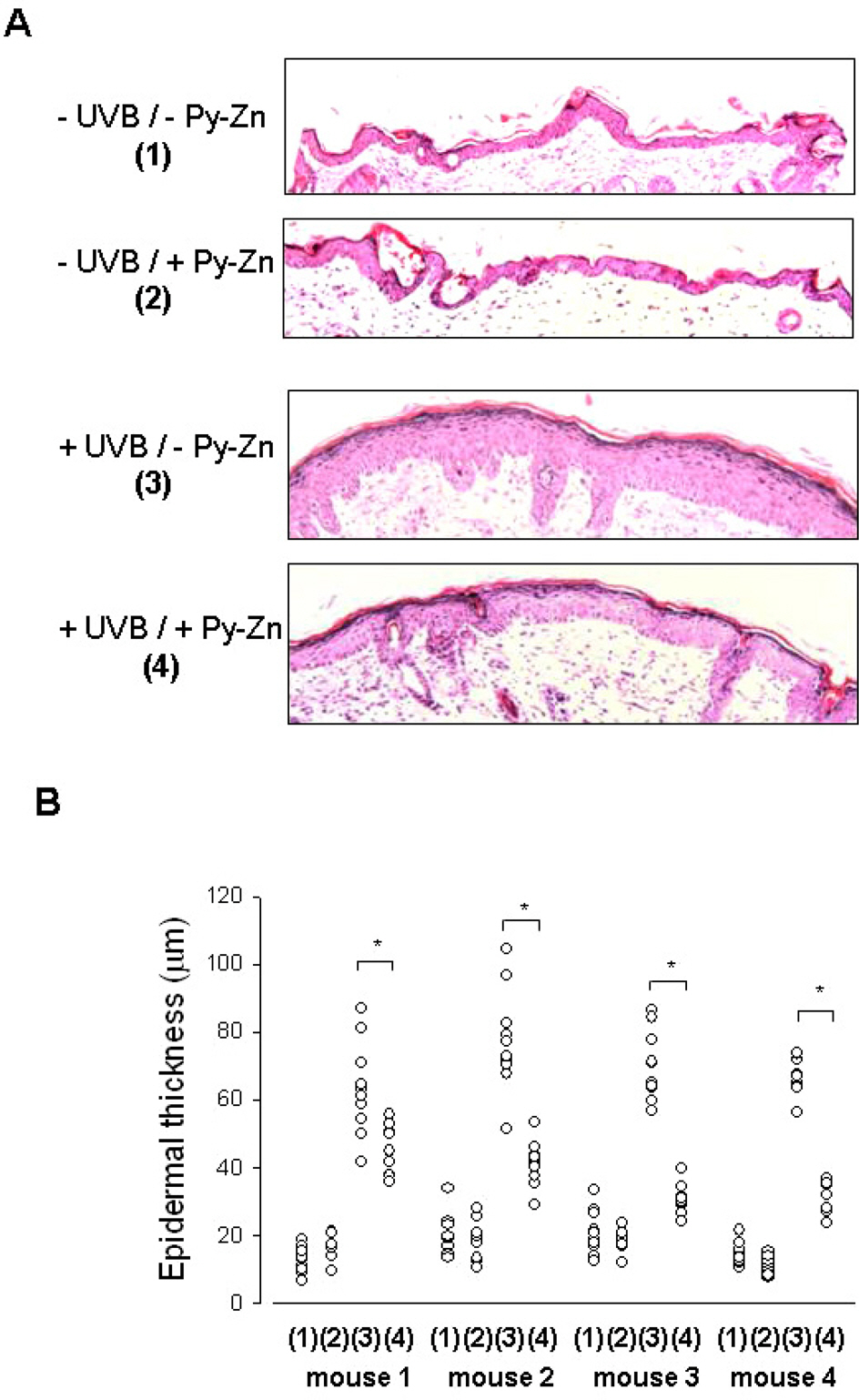
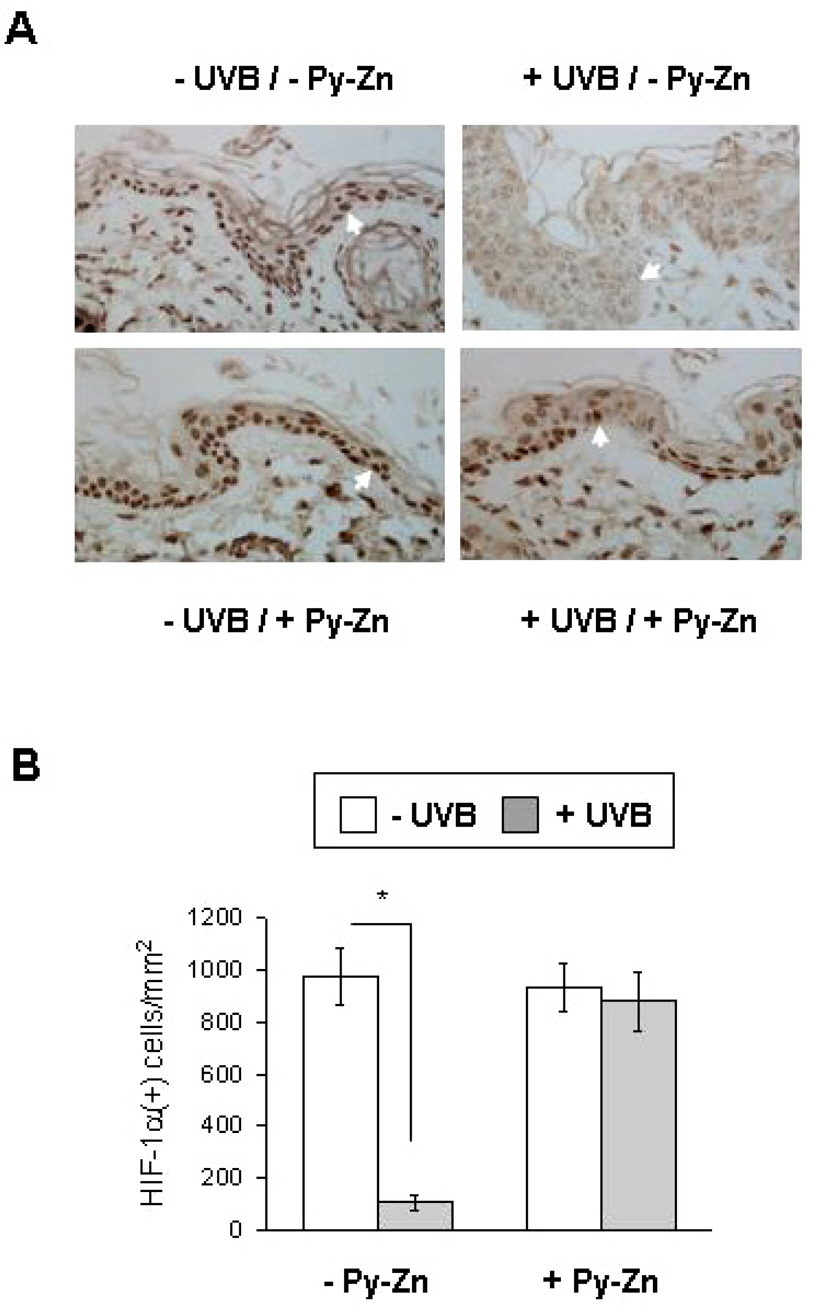
- Epidermal keratinocytes overgrow in response to ultraviolet-B (UVB), which may be associated with skin photoaging and cancer development. Recently, we found that HIF-1alpha controls the keratinocyte cell cycle and thereby contributes to epidermal homeostasis. A further study demonstrated that HIF-1alpha is down-regulated by UVB and that this process is involved in UVB-induced skin hyperplasia. Therefore, we hypothesized that the forced expression of HIF-1alpha in keratinocytes would prevent UVB-induced keratinocyte overgrowth. Among several agents known to induce HIF-1alpha, pyrithione-zinc (Py-Zn) overcame the UVB suppression of HIF-1alpha in cultured keratinocytes. Mechanistically, Py-Zn blocked the degradation of HIF-1alpha protein in keratinocytes, while it did not affect the synthesis of HIF-1alpha. Moreover, the p21 cell cycle inhibitor was down-regulated after UVB exposure, but was robustly induced by Py-Zn. In mice repeatedly irradiated with UVB, the epidermis became hyperplastic and HIF-1alpha disappeared from nuclei of epidermal keratinocytes. However, a cream containing Py-Zn effectively prevented the skin thickening and up-regulated HIF-1alpha to the normal level. These results suggest that Py-Zn is a potential agent to prevent UVB-induced photoaging and skin cancer development. This work also provides insight into a molecular target for treatment of UVB-induced skin diseases.
MeSH Terms
Figure
Reference
-
References
1. Melnikova VO, Ananthaswamy HN. Cellular and molecular events leading to the development of skin cancer. Mutat Res. 2005; 571:91–106.
Article2. Marrot L, Meunier JR. Skin DNA photodamage and its biological consequences. J Am Acad Dermatol. 2008; 58:S139–S148.
Article3. Ouhtit A, Muller HK, Davis DW, Ullrich SE, McConkey D, Ananthaswamy HN. Temporal events in skin injury and the early adaptive responses in ultraviolet-irradiated mouse skin. Am J Pathol. 2000; 156:201–207.
Article4. Lee JK, Kim JH, Nam KT, Lee SH. Molecular events associated with apoptosis and proliferation induced by ultraviolet-B radiation in the skin of hairless mice. J Dermatol Sci. 2003; 32:171–179.
Article5. El-Abaseri TB, Putta S, Hansen LA. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis. 2006; 27:225–231.
Article6. Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995; 270:1230–1237.
Article7. Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998; 12:149–162.8. Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1α induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004; 23:1949–1956.9. Cho YS, Bae JM, Chun YS, Chung JH, Jeon YK, Kim IS, Kim MS, Park JW. HIF-1α controls keratinocyte proliferation by up-regulating p 21(WAF1/Cip1). Biochim Biophys Acta. 2008; 1783:323–333.10. Cho YS, Kim CH, Park JW. Involvement of HIF-1α in UVB-induced epidermal hyperplasia. Mol Cells. 2009; 28:537–543.11. Chun YS, Choi E, Kim GT, Lee MJ, Lee MJ, Lee SE, Kim MS, Park JW. Zinc induces the accumulation of hypoxia-inducible factor (HIF)-1α, but inhibits the nuclear translocation of HIF-1β, causing HIF-1 inactivation. Biochem Biophys Res Commun. 2000; 268:652–656.12. Lim JH, Park JW, Kim MS, Park SK, Johnson RS, Chun YS. Bafilomycin induces the p21-mediated growth inhibition of cancer cells under hypoxic conditions by expressing hypoxia-inducible factor-1α. Mol Pharmacol. 2006; 70:1856–1865.13. Cho H, Lee HY, Ahn DR, Kim SY, Kim S, Lee KB, Lee YM, Park H, Yang EG. Baicalein induces functional hypoxia-inducible factor-1α and angiogenesis. Mol Pharmacol. 2008; 74:70–81.14. Weinreb O, Amit T, Youdim MB. A novel approach of proteomics and transcriptomics to study the mechanism of action of the antioxidant-iron chelator green tea polyphenol (–)-epigallo-catechin-3-gallate. Free Radic Biol Med. 2007; 43:546–556.
Article15. Park SE, Park JW, Cho YS, Ryu JH, Paick JS, Chun YS. HIF-1α promotes survival of prostate cells at a high zinc environment. Prostate. 2007; 67:1514–1523.16. Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxiainducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998; 95:7987–7992.17. Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004; 5:343–354.
Article18. Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002; 22:7004–7014.19. Lang KJ, Kappel A, Goodall GJ. Hypoxia-inducible factor-1α mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol Biol Cell. 2002; 13:1792–1801.20. Arsham AM, Plas DR, Thompson CB, Simon MC. Phosphatidylinositol 3-kinase/Akt signaling is neither required for hypoxic stabilization of HIF-1α nor sufficient for HIF-1-dependent target gene transcription. J Biol Chem. 2002; 277:15162–15170.21. Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1α-degradative pathway. J Biol Chem. 2002; 277:29936–29944.22. Luo W, Zhong J, Chang R, Hu H, Pandey A, Semenza GL. Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1α but Not HIF-2α. J Biol Chem. 2010; 285:3651–3663.23. Pierard-Franchimont C, Goffin V, Decroix J, Pierard GE. A multicenter randomized trial of ketoconazole 2% and zinc pyrithione 1% shampoos in severe dandruff and seborrheic dermatitis. Skin Pharmacol Appl Skin Physiol. 2002; 15:434–441.24. Bailey P, Arrowsmith C, Darling K, Dexter J, Eklund J, Lane A, Little C, Murray B, Scott A, Williams A, Wilson D. A doubleblind randomized vehicle-controlled clinical trial investigating the effect of ZnPTO dose on the scalp vs. antidandruff efficacy and antimycotic activity. Int J Cosmet Sci. 2003; 25:183–188.
Article25. Guthery E, Seal LA, Anderson EL. Zinc pyrithione in alcohol-based products for skin antisepsis: persistence of antimicrobial effects. Am J Infect Control. 2005; 33:15–22.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Relationship between Expression of Hypoxia Inducible Factor-1alpha or Vascular Endothelial Growth Factor and Histopathological Characteristics in Human Transitional Bladder Cancer
- The Relationship between Expression of Hypoxia Inducible Factor-1alpha or Vascular Endothelial Growth Factor and Histopathological Characteristics in Human Renal Cell Carcinoma
- HIF-1alpha Upregulation due to Depletion of the Free Ubiquitin Pool
- The Clinicopathological Significance of Tissue Levels of Hypoxia-Inducible Factor-1alpha and Vascular Endothelial Growth Factor in Gastric Cancer
- Phospholipase D activates HIF-1-VEGF pathway via phosphatidic acid