Korean J Obstet Gynecol.
2010 Oct;53(10):905-914. 10.5468/kjog.2010.53.10.905.
The effect of granulocyte-macrophage-stimulating factor on the gene expression in human trophoblast of early pregnancy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Eulji University School of Medicine, Daejeon, Korea. pwi3110@eulji.ac.kr
- 2Life Science Institute, Eulji University School of Medicine, Daejeon, Korea.
- 3Laboratory of Molecular Oncology, Cheil General Hospital, Kwandong University College of Medicine, Seoul, Korea.
- 4Department of Obstetrics and Gynecology, Ewha Woman's University School of Medicine, Seoul, Korea.
- KMID: 1453755
- DOI: http://doi.org/10.5468/kjog.2010.53.10.905
Abstract
OBJECTIVE
Granulocyte-macrophage colony-stimulating factor (GM-CSF) facilitates mammalian embryonic development and implantation. However, its biological function after implantation is not elucidated. The aim of this study is to assess the changes of gene expression by GM-CSF in human trophoblast obtained in early pregnancy.
METHODS
Human trophoblast obtained in early pregnancy was cultured with or without GM-CSF. The difference of gene expression was evaluated with microarray and selected genes were reevaluated with real-time reverse transcription-polymerase chain reaction (RT-PCR).
RESULTS
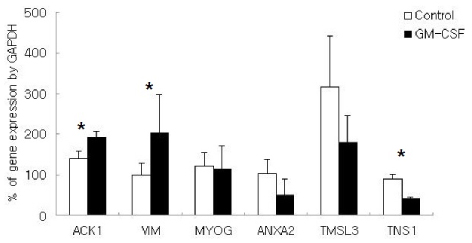
Microarray analysis revealed that the expressions of 468 genes were increased while those of 40 genes were decreased by GM-CSF. These genes were evaluated according to the known biologic pathways. The regulation of actin cytoskeleton and focal adhesion pathways were mostly influenced by GM-CSF. Annexin A2, thymosin-like 3, vimentin, myogenin, ACK1, and tensin1 genes were selected for real-time RT-PCR. The increased expressions of of vimentin and ACK1, and decreased expressions of tensin1 were confirmed by real-time RT-PCR.
CONCLUSION
GM-CSF activates focal adhesion pathway in human trophoblast by increasing the expression of vimentin and ACK1, and decreasing the expression of tensin1.
MeSH Terms
Figure
Reference
-
1. Thie M, Denker HW. In vitro studies on endometrial adhesiveness for trophoblast: cellular dynamics in uterine epithelial cells. Cells Tissues Organs. 2002. 172:237–252.2. Harris LK, Jones CJ, Aplin JD. Adhesion molecules in human trophoblast-a review. II. extravillous trophoblast. Placenta. 2009. 30:299–304.3. Robertson SA, Seamark RF. Granulocyte macrophage colony stimulating factor (GM-CSF) in the murine reproductive tract: stimulation by seminal factors. Reprod Fertil Dev. 1990. 2:359–368.4. Jokhi PP, King A, Loke YW. Production of granulocyte-macrophage colony-stimulating factor by human trophoblast cells and by decidual large granular lymphocytes. Hum Reprod. 1994. 9:1660–1669.5. Saito S, Nishikawa K, Morii T, Enomoto M, Narita N, Motoyoshi K, et al. Cytokine production by CD16-CD56bright natural killer cells in the human early pregnancy decidua. Int Immunol. 1993. 5:559–563.6. Jokhi PP, King A, Jubinsky PT, Loke YW. Demonstration of the low affinity alpha subunit of the granulocyte-macrophage colony-stimulating factor receptor (GM-CSF-R alpha) on human trophoblast and uterine cells. J Reprod Immunol. 1994. 26:147–164.7. Garcia-Lloret MI, Morrish DW, Wegmann TG, Honore L, Turner AR, Guilbert LJ. Demonstration of functional cytokine-placental interactions: CSF-1 and GM-CSF stimulate human cytotrophoblast differentiation and peptide hormone secretion. Exp Cell Res. 1994. 214:46–54.8. Kim DH, Ko DS, Lee HC, Lee HJ, Kang HG, Kim TJ, et al. Effect of GM-CSF on the embryonic development and the expression of implantation related genes of mouse embryos. Korean J Fertil Steril. 2002. 29:83–90.9. Kim HJ, Kim HM, Kim JL, Shin JH, Hong SY, Park EJ, et al. The effect of GM-CSF on the expression of implantation-related genes in mouse embryo. Korean J Obstet Gynecol. 2008. 51:199–211.10. Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, et al. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002. 30:e15.11. Robertson SA, Sjoblom C, Jasper MJ, Norman RJ, Seamark RF. Granulocyte-macrophage colony-stimulating factor promotes glucose transport and blastomere viability in murine preimplantation embryos. Biol Reprod. 2001. 64:1206–1215.12. Robertson SA, Roberts CT, Farr KL, Dunn AR, Seamark RF. Fertility impairment in granulocytemacrophage colony-stimulating factor-deficient mice. Biol Reprod. 1999. 60:251–261.13. Sjoblom C, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor (GM-CSF) acts independently of the beta common subunit of the GM-CSF receptor to prevent inner cell mass apoptosis in human embryos. Biol Reprod. 2002. 67:1817–1823.14. Park WI, Kwon HC, Kim DH, Kang HK, Kim MK, Lee HC, et al. The effect of GM-CSF supplementation in culture medium in the human IVF programs. Korean J Fertil Steril. 2001. 28:161–168.15. Armstrong DT, Chaouat G. Effects of lymphokines and immune complexes on murine placental cell growth in vitro. Biol Reprod. 1989. 40:466–474.16. Olson MF, Sahai E. The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis. 2009. 26:273–287.17. Chakraborty C, Gleeson LM, McKinnon T, Lala PK. Regulation of human trophoblast migration and invasiveness. Can J Physiol Pharmacol. 2002. 80:116–124.18. Rescher U, Ludwig C, Konietzko V, Kharitonenkov A, Gerke V. Tyrosine phosphorylation of annexin A2 regulates Rho-mediated actin rearrangement and cell adhesion. J Cell Sci. 2008. 121:2177–2185.19. Pomies P, Pashmforoush M, Vegezzi C, Chien KR, Auffray C, Beckerle MC. The cytoskeleton-associated PDZ-LIM protein, ALP, acts on serum response factor activity to regulate muscle differentiation. Mol Biol Cell. 2007. 18:1723–1733.20. Komati H, Naro F, Mebarek S, De Arcangelis V, Adamo S, Lagarde M, et al. Phospholipase D is involved in myogenic differentiation through remodeling of actin cytoskeleton. Mol Biol Cell. 2005. 16:1232–1244.21. Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004. 1692:103–119.22. Pollheimer J, Knofler M. Signalling pathways regulating the invasive differentiation of human trophoblasts: a review. Placenta. 2005. 26:SupplA. S21–S30.23. Clauss IM, Wathelet MG, Szpirer J, Islam MQ, Levan G, Szpirer C, et al. Human thymosin-beta 4/6-26 gene is part of a multigene family composed of seven members located on seven different chromosomes. Genomics. 1991. 9:174–180.24. Wang WS, Chen PM, Hsiao HL, Ju SY, Su Y. Overexpression of the thymosin beta-4 gene is associated with malignant progression of SW480 colon cancer cells. Oncogene. 2003. 22:3297–3306.25. Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994. 79:679–694.26. Gonzales M, Weksler B, Tsuruta D, Goldman RD, Yoon KJ, Hopkinson SB, et al. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol Biol Cell. 2001. 12:85–100.27. Tsuruta D, Jones JC. The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J Cell Sci. 2003. 116:4977–4984.28. Wei J, Xu G, Wu M, Zhang Y, Li Q, Liu P, et al. Overexpression of vimentin contributes to prostate cancer invasion and metastasis via src regulation. Anticancer Res. 2008. 28:327–334.29. Zhao Y, Yan Q, Long X, Chen X, Wang Y. Vimentin affects the mobility and invasiveness of prostate cancer cells. Cell Biochem Funct. 2008. 26:571–577.30. Manser E, Leung T, Salihuddin H, Tan L, Lim L. A non-receptor tyrosine kinase that inhibits the GTPase activity of p21cdc42. Nature. 1993. 363:364–367.31. Howlin J, Rosenkvist J, Andersson T. TNK2 preserves epidermal growth factor receptor expression on the cell surface and enhances migration and invasion of human breast cancer cells. Breast Cancer Res. 2008. 10:R36.32. Thelemann A, Petti F, Griffin G, Iwata K, Hunt T, Settinari T, et al. Phosphotyrosine signaling networks in epidermal growth factor receptor overexpressing squamous carcinoma cells. Mol Cell Proteomics. 2005. 4:356–376.33. Martuszewska D, Ljungberg B, Johansson M, Landberg G, Oslakovic C, Dahlback B, et al. Tensin3 is a negative regulator of cell migration and all four Tensin family members are downregulated in human kidney cancer. PLoS One. 2009. 4:e4350.34. Lo SH, Yu QC, Degenstein L, Chen LB, Fuchs E. Progressive kidney degeneration in mice lacking tensin. J Cell Biol. 1997. 136:1349–1361.35. Eto M, Kirkbride J, Elliott E, Lo SH, Brautigan DL. Association of the tensin N-terminal protein-tyrosine phosphatase domain with the alpha isoform of protein phosphatase-1 in focal adhesions. J Biol Chem. 2007. 282:17806–17815.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of combination gene therapy with herpes simplex virus thymidine kinase suicidal gene and granulocyte-macrophage colony-stimulating factor gene in murine melanoma model
- The effects on the production of platelet activating factor in the cultured human endothelial cells by interleukin-6 and granulocyte macrophage colony stimulating factor
- Preliminary study of antithymocyte or antilymphocyte globulin, cyclosporine-A and recombinant human granulocyte macrophage colony stimulating factors for patients with aplastic anemia
- A study of the effects of GM-CSF incubation on long-term human bone marrow cultures
- Expression of Epidermal Growth Factor, Transforming Growth Factor-alphaand Epidermal Growth Factor Receptor in Human Trophoblast and Decidua