J Bacteriol Virol.
2011 Mar;41(1):9-18. 10.4167/jbv.2011.41.1.9.
Effect of Weissella cibaria on Fusobacterium nucleatum-induced Interleukin-6 and Interleukin-8 Production in KB Cells
- Affiliations
-
- 1Department of Microbiology, School of Medicine, Chonnam National University, Gwangju, Korea. joh@chonnam.ac.kr
- 2Dental Science Research Institute, Chonnam National University, Gwangju, Korea.
- KMID: 1449858
- DOI: http://doi.org/10.4167/jbv.2011.41.1.9
Abstract
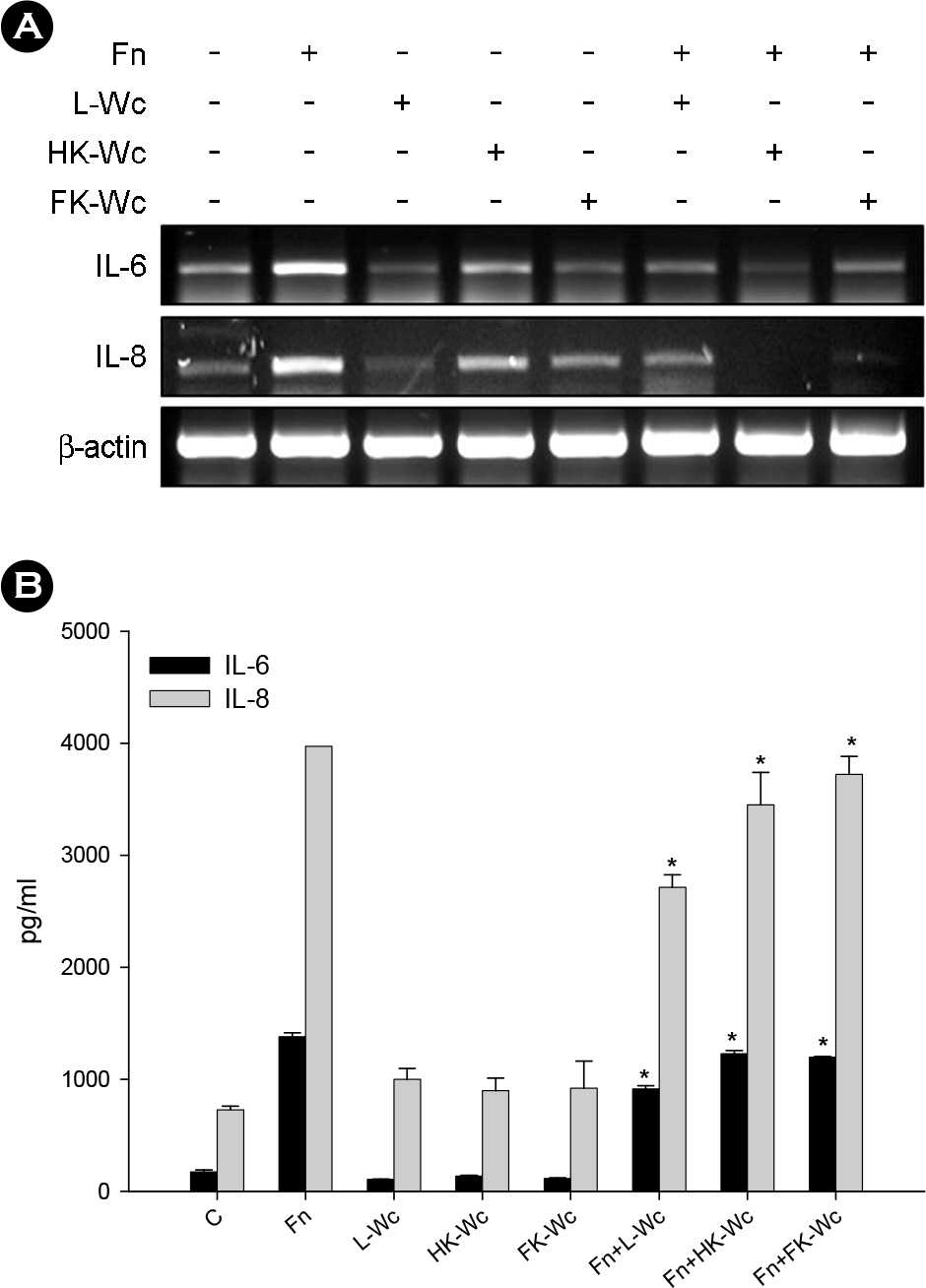
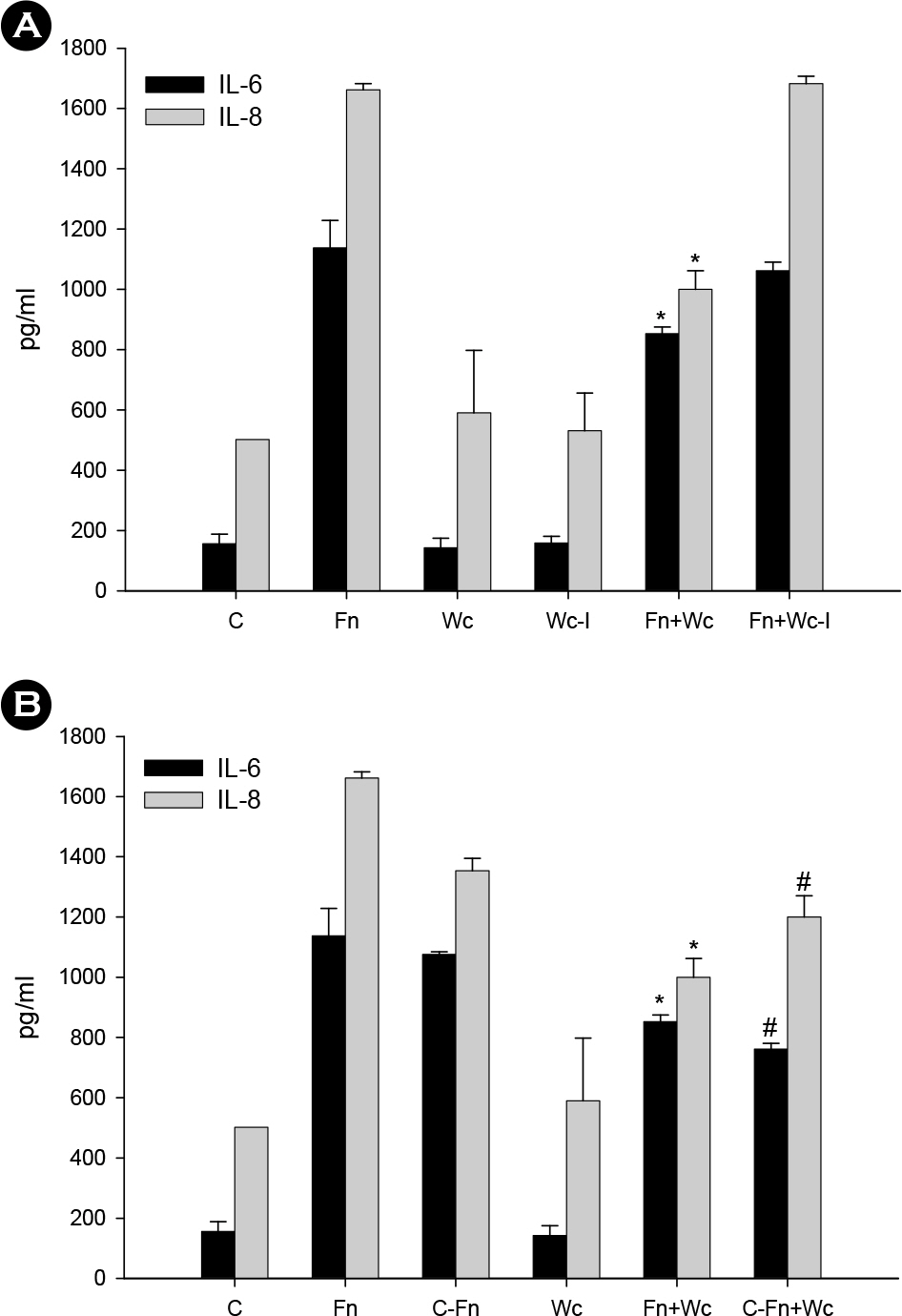
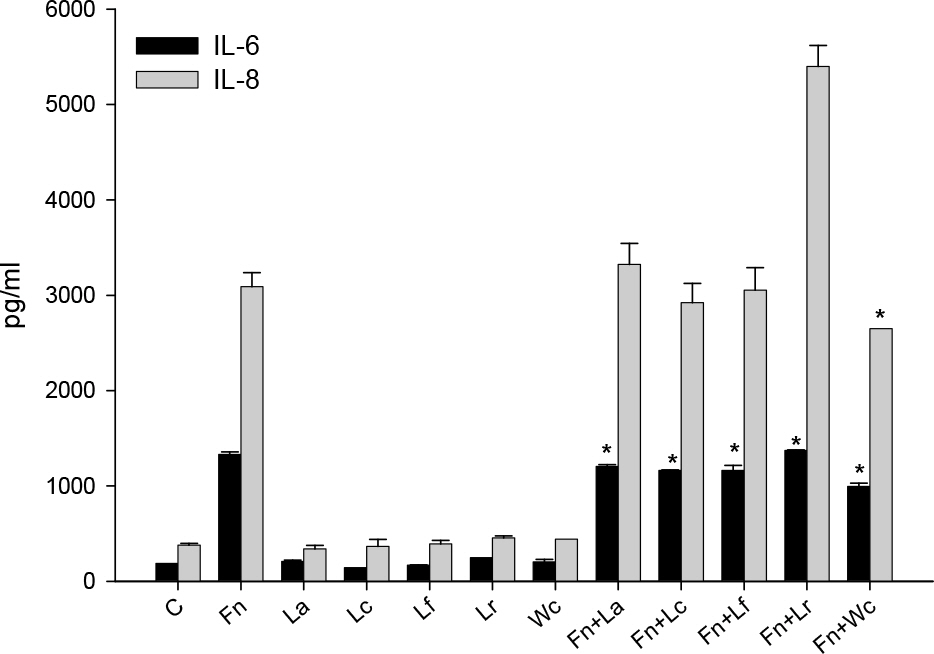
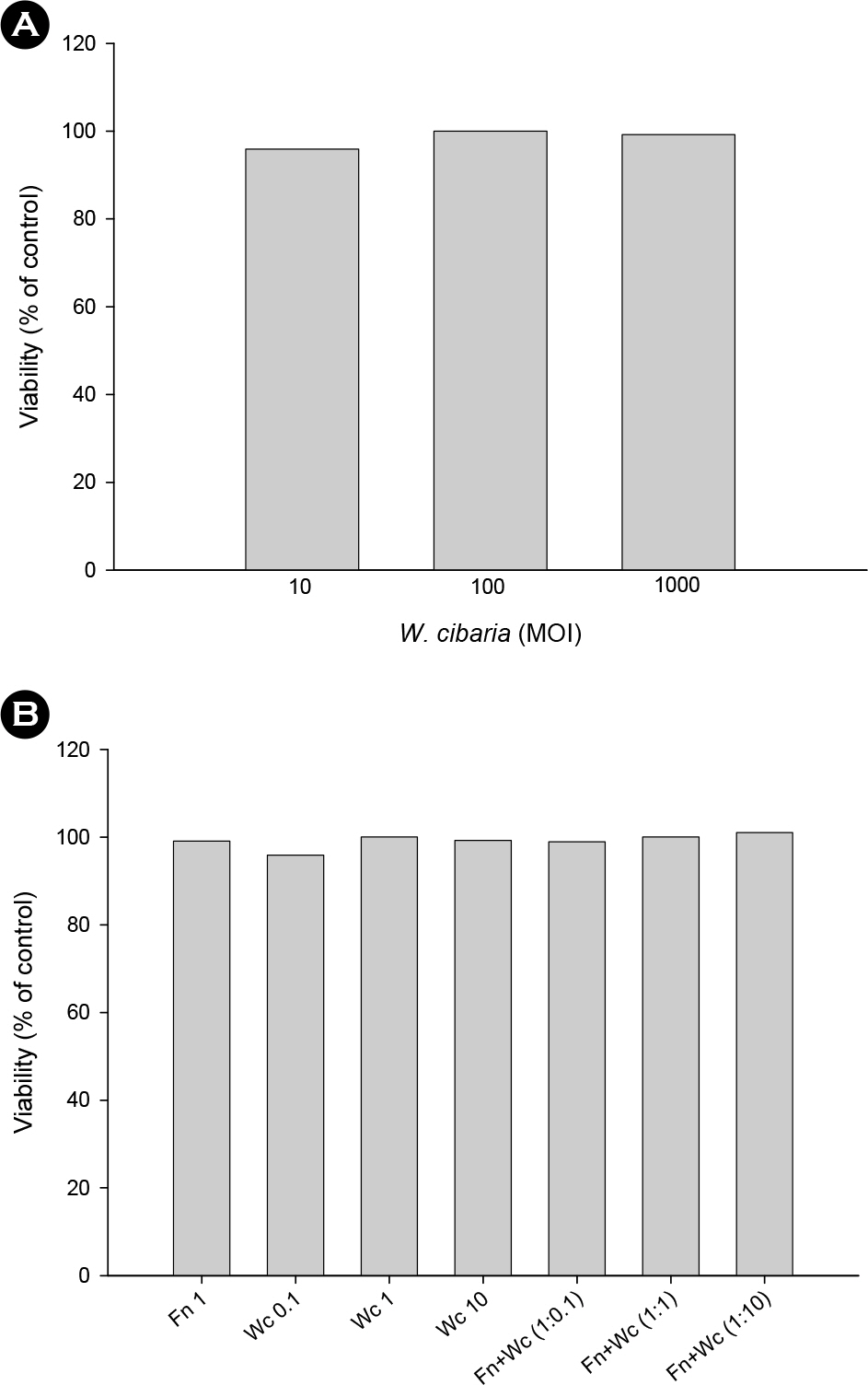
- Oral microorganisms, including pathogens together with commensals, interact with oral epithelial cells, which can lead to the activation and expression of a variety of inflammatory mediators in epithelial cells. Fusobacterium nucleatum is a filamentous human pathogen that is strongly associated with periodontal diseases. Our previous data suggest that Weissella cibaria, an oral commensal, inhibits the proliferation of periodontopathic bacteria including F. nucleatum. The aim of this study was to examine the effects of W. cibaria on the inflammatory mediators, interleukin (IL)-6 and IL-8, in KB cells stimulated by F. nucleatum. In a reverse transcription-polymerase chain reaction and an enzyme-linked immunosorbent assay, live F. nucleatum alone induced high levels of gene expression and protein release of IL-6 and IL-8, whereas W. cibaria alone did not induce IL-6 and IL-8 responses in KB cells. W. cibaria dose-dependently inhibited the increases of the IL-6 and IL-8 gene expression as well as IL-6 protein level in KB cells which was induced by F. nucleatum. Bacterial viability and its coaggregation with F. nucleatum are not essential in the inhibitory effect of W. cibaria. Visible effects of W. cibaria on the attachment and invasion of KB cells by F. nucleatum were observed. In conclusion, W. cibaria may exert immunomodulatory effects on the IL-6 and IL-8 responses to F. nucleatum-activated KB cells.
MeSH Terms
Figure
Reference
-
1). Haffajee AD., Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994. 5:78–111.
Article2). Slots J., Ting M. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontol 2000. 1999. 20:82–121.3). Haffajee AD., Teles RP., Socransky SS. Association of Eubacterium nodatum and Treponema denticola with human periodontitis lesions. Oral Microbiol Immunol. 2006. 21:269–82.4). Bolstad AI., Jensen HB., Bakken V. Taxonomy, biology, and periodontal aspects of Fusobacterium nucleatum. Clin Microbiol Rev. 1996. 9:55–71.5). Han YW., Shi W., Huang GT., Kinder Haake S., Park NH., Kuramitsu H, et al. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect Immun. 2000. 68:3140–6.6). Kagnoff MF., Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997. 100:6–10.
Article7). Okada H., Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998. 9:248–66.
Article8). Birkedal-Hansen H. Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res. 1993. 28:500–10.
Article9). Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990. 8:253–78.
Article10). Irwin CR., Myrillas TT. The role of IL-6 in the pathogenesis of periodontal disease. Oral Dis. 1998. 4:43–7.
Article11). Yumoto H., Nakae H., Fujinaka K., Ebisu S., Matsuo T. Interleukin-6 (IL-6) and IL-8 are induced in human oral epithelial cells in response to exposure to periodontopathic Eikenella corrodens. Infect Immun. 1999. 67:384–94.12). Sfakianakis A., Barr CE., Kreutzer D. Mechanisms of Actinobacillus actinomycetemcomitans-induced expression of interleukin-8 in gingival epithelial cells. J Periodontol. 2001. 72:1413–9.13). Takashiba S., Naruishi K., Murayama Y. Perspective of cytokine regulation for periodontal treatment: fibroblast biology. J Periodontol. 2003. 74:103–10.
Article14). Frieling J., Mulder JA., Hendriks T., Curfs JH., van der Linden CJ., Sauerwein RW. Differential induction of pro- and anti-inflammatory cytokines in whole blood by bacteria: effects of antibiotic treatment. Antimicrob Agents Chemother. 1997. 41:1439–43.
Article15). Neish AS., Gewirtz AT., Zeng H., Young AN., Hobert ME., Karmali V, et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000. 289:1560–3.16). Kelly D., Campbell JI., King TP., Grant G., Jansson EA., Coutts AG, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004. 5:104–12.17). Bjorkroth KJ., Schillinger U., Geisen R., Weiss N., Hoste B., Holzapfel WH, et al. Taxonomic study of Weissella confusa and description of Weissella cibaria sp. nov., detected in food and clinical samples. Int J Syst Evol Microbiol. 2002. 52:141–8.18). Kang MS., Chung J., Kim SM., Yang KH., Oh JS. Effect of Weissella cibaria isolates on the formation of Streptococcus mutans biofilm. Caries Res. 2006. 40:418–25.19). Kang MS., Kim BG., Chung J., Lee HC., Oh JS. Inhibitory effect of Weissella cibaria isolates on the production of volatile sulphur compounds. J Clin Periodontol. 2006. 33:226–32.20). Kang MS., Lim HS., Kim SM., Lim YJ., Lee HC., Oh JS. Quantitative analysis of Weissella cibaria against periodontopathic bacteria by real-time PCR. J Bacteriol Virol. 2009. 39:295–305.21). Kang MS., Na HS., Oh JS. Coaggregation ability of Weissella cibaria isolates with Fusobacterium nucleatum and their adhesiveness to epithelial cells. FEMS Microbiol Lett. 2005. 253:323–9.22). Edwards AM., Grossman TJ., Rudney JD. Fusobacterium nucleatum transports noninvasive Streptococcus cristatus into human epithelial cells. Infect Immun. 2006. 74:654–62.23). Kang IC., Kuramitsu HK. Induction of monocyte chemoattractant protein-1 by Porphyromonas gingivalis in human endothelial cells. FEMS Immunol Med Microbiol. 2002. 34:311–7.24). Na YJ., Jeon YJ., Suh JH., Kang JS., Yang KH., Kim HM. Suppression of IL-8 gene expression by radicicol is mediated through the inhibition of ERK1//2 and p38 signaling and negative regulation of NF-kappaB and AP-1. Int Immunopharmacol. 2001. 1:1877–87.25). Deshpande RG., Khan MB., Genco CA. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998. 66:5337–43.26). Walker C., Ratliff D., Muller D., Mandell R., Socransky SS. Medium for selective isolation of Fusobacterium nucleatum from human periodontal pockets. J Clin Microbiol. 1979. 10:844–9.27). Kang MS., Oh JS., Kang IC., Hong SJ., Choi CH. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J Microbiol. 2008. 46:744–50.
Article28). Kornman KS., Page RC., Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997. 14:33–53.
Article29). Kjeldsen M., Holmstrup P., Bendtzen K. Marginal periodontitis and cytokines: a review of the literature. J Periodontol. 1993. 64:1013–22.
Article30). Wilson M., Reddi K., Henderson B. Cytokine-inducing components of periodontopathic bacteria. J Periodontal Res. 1996. 31:393–407.31). Tonetti MS., Imboden MA., Gerber L., Lang MP., Laissue J., Mueller C. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun. 1994. 62:4005–14.
Article32). Gamonal J., Acevedo A., Bascones A., Jorge O., Silva A. Characterization of cellular infiltrate, detection of chemokine receptor CCR5 and interleukin-8 and RANTES chemokines in adult periodontitis. J Periodontal Res. 2001. 36:194–203.
Article33). Sansonetti P. War and peace at mucosal surfaces. Nat Rev Immunol. 2004. 4:953–64.
Article34). Zhang L., Li N., Caicedo R., Neu J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha induced interleukin-8 production in Caco-2 cells. J Nutr. 2005. 135:1752–6.35). Huang GT., Kim D., Lee JK., Kuramitsu HK., Haake SK. Interleukin-8 and intercellular adhesion molecule 1 regulation in oral epithelial cells by selected periodontal bacteria: multiple effects of Porphyromonas gingivalis via antagonistic mechanisms. Infect Immun. 2001. 69:1364–72.36). Erdèlyi K., Kiss A., Bakondi E., Bai P., Szabó C., Gergely P, et al. Gallotannin inhibits the expression of chemokines and inflammatory cytokines in A549 cells. Mol Pharmacol. 2005. 68:895–904.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adhesion of Weissella cibaria to the Epithelial Cells and Factors Affecting its Adhesion
- Effect of Natural Extracts on Oral Care Probiotics Weissella cibaria CMU and Periodontal Pathogens
- Quantitative Analysis of Weissella cibaria against Periodontopathic Bacteria by Real-time PCR
- Immunomodulatory effects mixed with Weissella cibaria JW15 and Black soybean (Glycine max (L.) Merr.) Extract
- Fusobacterium nucleatum stimulates dental pulp cells to produce prostaglandin E 2 via mitogen-activated protein kinases activation