Korean J Physiol Pharmacol.
2011 Jun;15(3):179-187. 10.4196/kjpp.2011.15.3.179.
CD40 Co-stimulation Inhibits Sustained BCR-induced Ca2+ Signaling in Response to Long-term Antigenic Stimulation of Immature B Cells
- Affiliations
-
- 1Department of Physiology, SBRI, Sungkyunkwan University School of Medicine, Suwon 440-746, Korea. tongmkang@skku.edu
- 2Department of Molecular Cell Biology, SBRI, Sungkyunkwan University School of Medicine, Suwon 440-746, Korea.
- 3Department of Physiology, Seoul National University College of Medicine, Seoul 110-799, Korea.
- KMID: 1448922
- DOI: http://doi.org/10.4196/kjpp.2011.15.3.179
Abstract
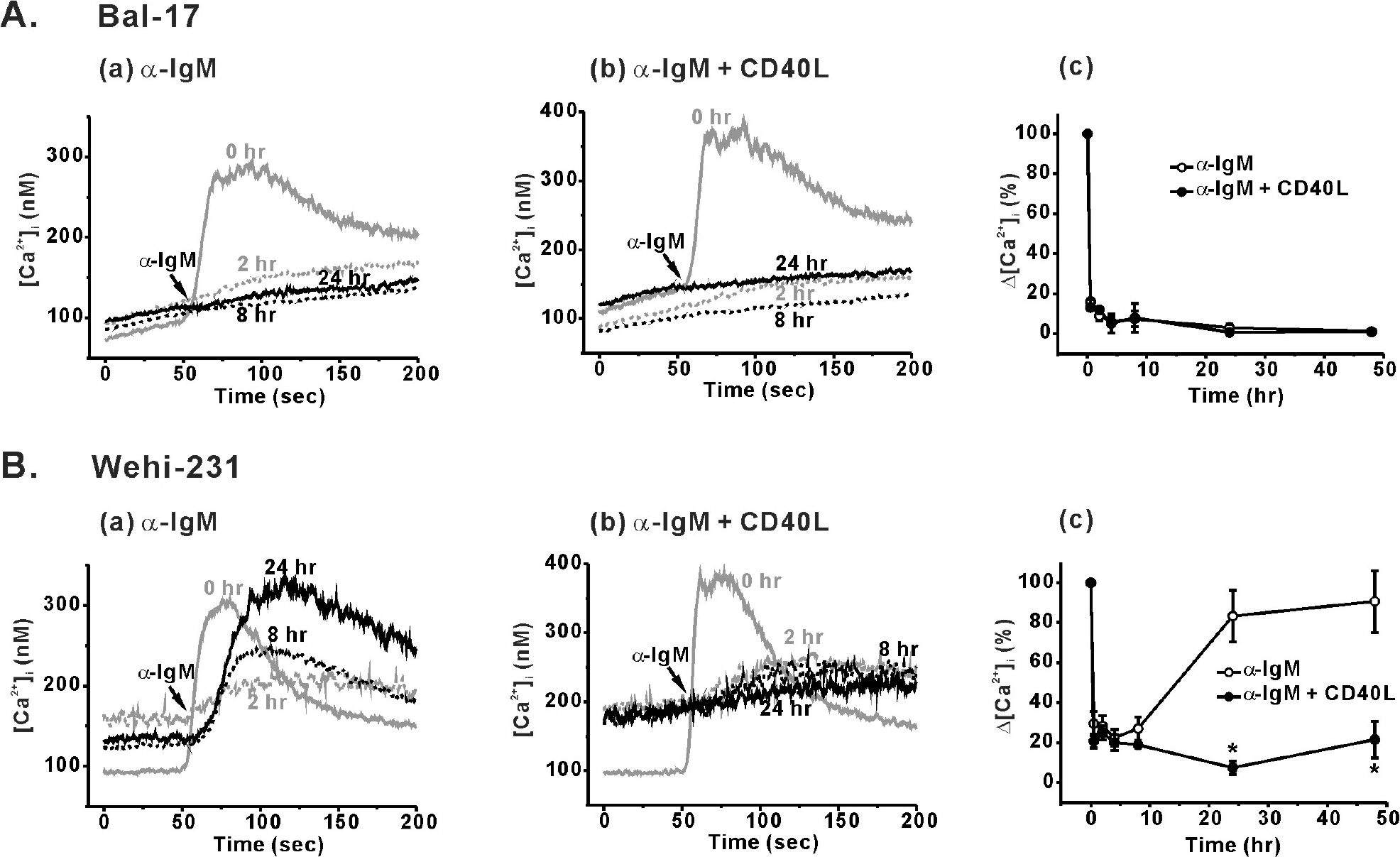
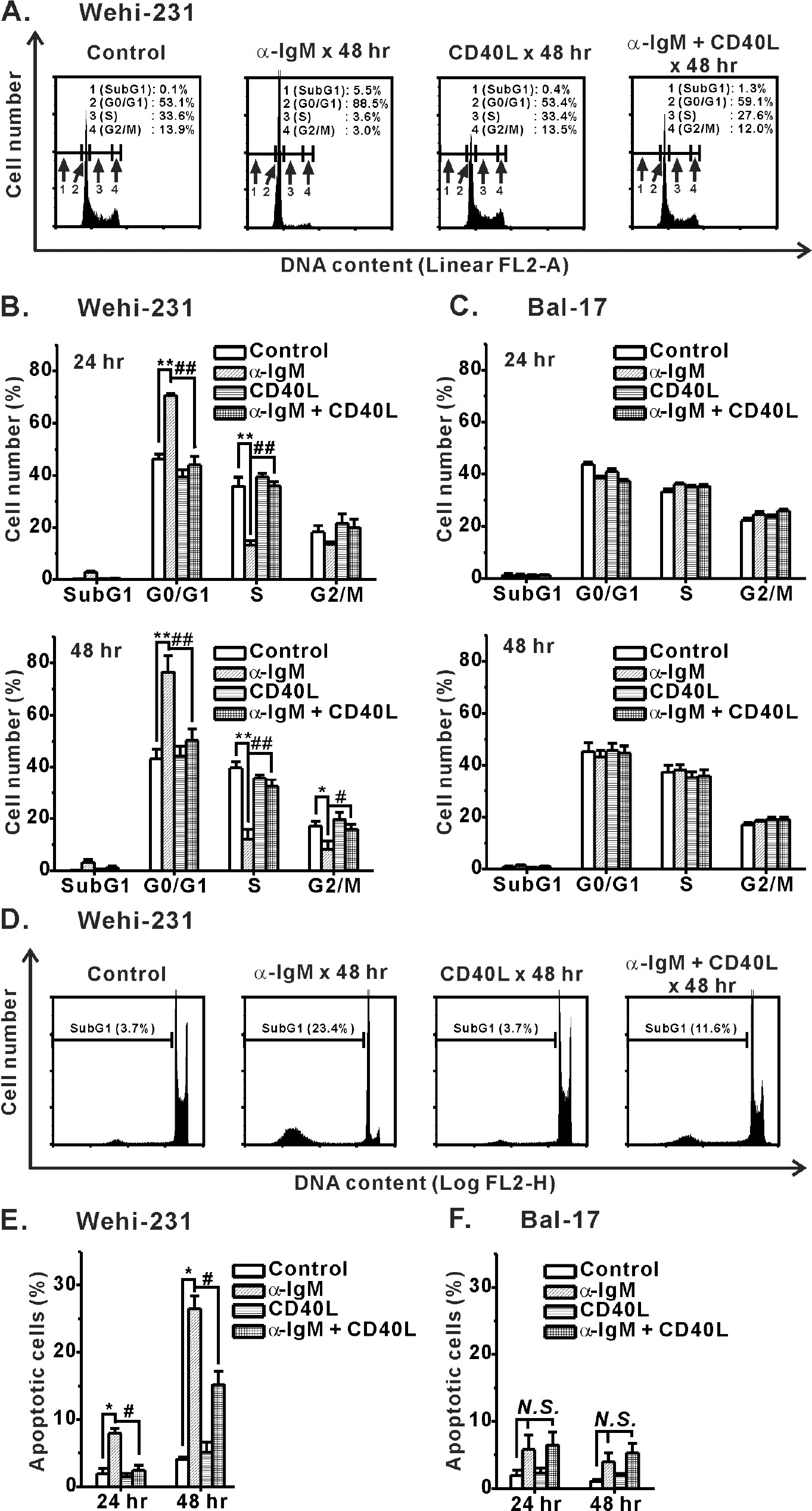
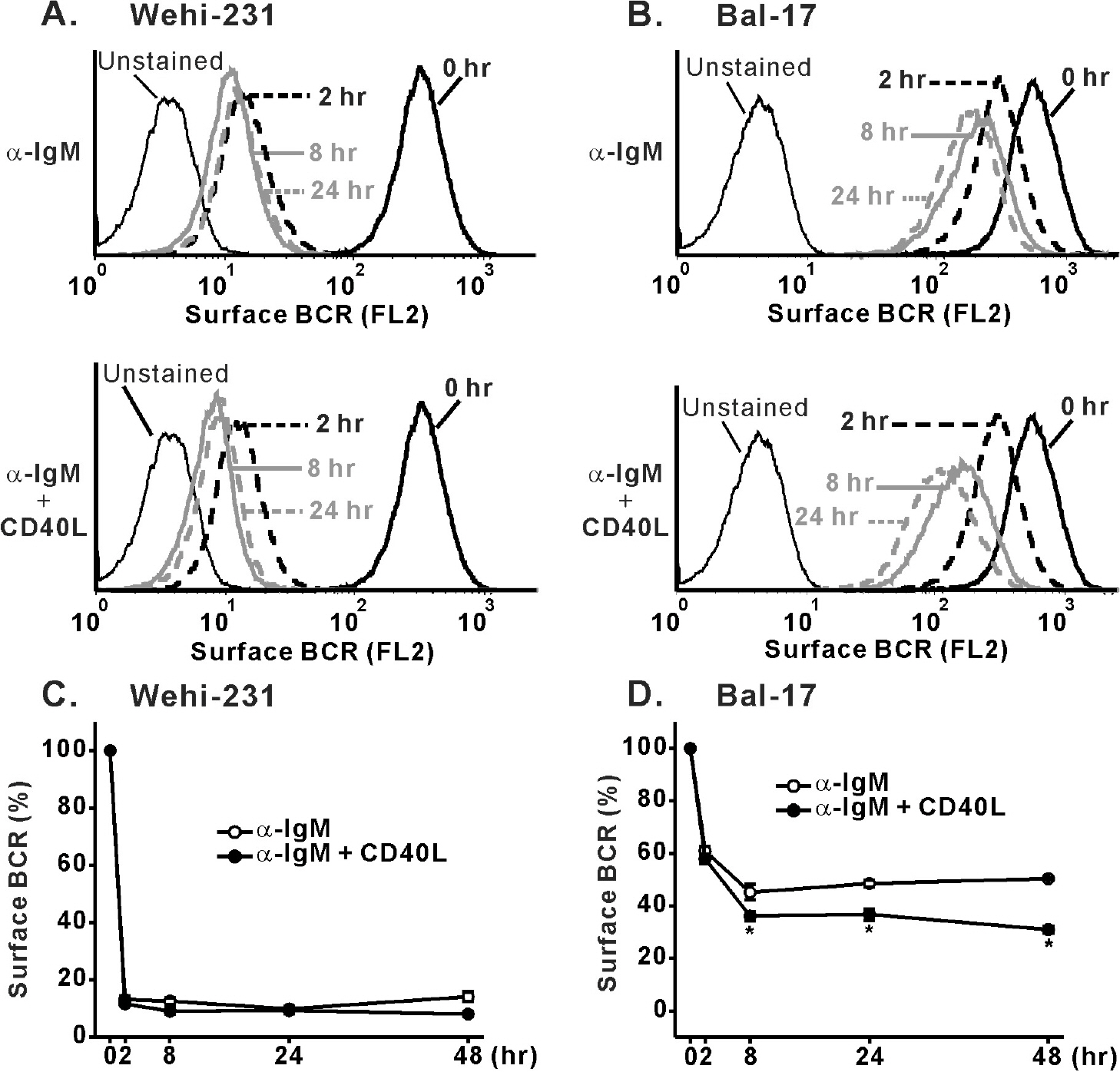
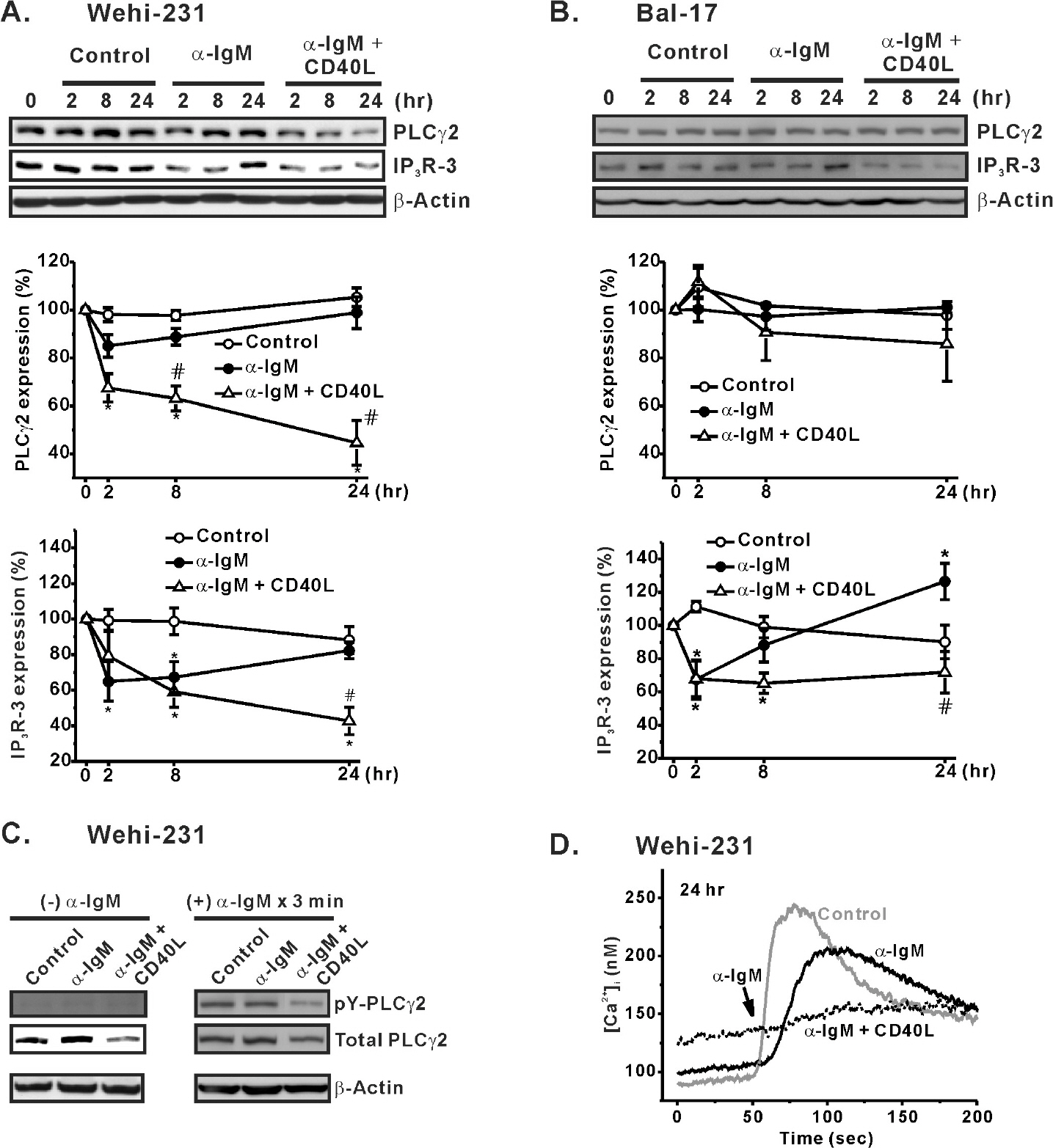
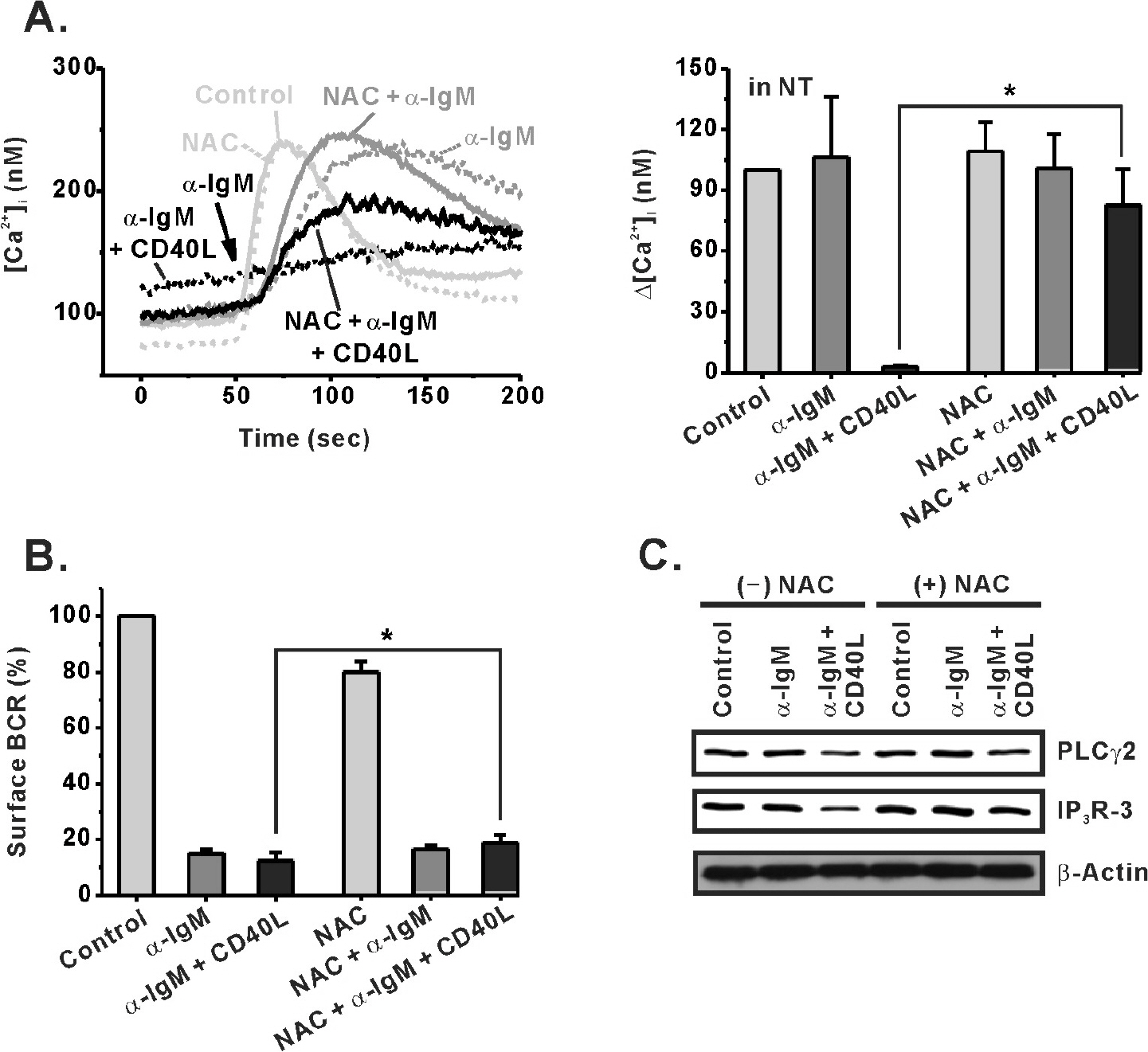
- Regulation of B cell receptor (BCR)-induced Ca2+ signaling by CD40 co-stimulation was compared in long-term BCR-stimulated immature (WEHI-231) and mature (Bal-17) B cells. In response to long-term pre-stimulation of immature WEHI-231 cells to alpha-IgM antibody (0.5~48 hr), the initial transient decrease in BCR-induced [Ca2+]i was followed by spontaneous recovery to control level within 24 hr. The recovery of Ca2+ signaling in WEHI-231 cells was not due to restoration of internalized receptor but instead to an increase in the levels of PLCgamma2 and IP3R-3. CD40 co-stimulation of WEHI-231 cells prevented BCR-induced cell cycle arrest and apoptosis, and it strongly inhibited the recovery of BCR-induced Ca2+ signaling. CD40 co-stimulation also enhanced BCR internalization and reduced expression of PLCgamma2 and IP3R-3. Pre-treatment of WEHI-231 cells with the antioxidant N-acetyl-L-cysteine (NAC) strongly inhibited CD40-mediated prevention of the recovery of Ca2+ signaling. In contrast to immature WEHI-231 cells, identical long-term alpha-IgM pre-stimulation of mature Bal-17 cells abolished the increase in BCR-induced [Ca2+]i, regardless of CD40 co-stimulation. These results suggest that CD40-mediated signaling prevents antigen-induced cell cycle arrest and apoptosis of immature B cells through inhibition of sustained BCR-induced Ca2+ signaling.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002; 2:945–956.
Article2. Scharenberg AM, Humphries LA, Rawlings DJ. Calcium signalling and cell-fate choice in B cells. Nat Rev Immunol. 2007; 7:778–789.
Article3. Koncz G, Bodor C, Kövesdi D, Gáti R, Sármay G. BCR mediated signal transduction in immature and mature B cells. Immunol Lett. 2002; 82:41–49.
Article4. Harwood NE, Batista FD. New insights into the early molecular events underlying B cell activation. Immunity. 2008; 28:609–619.
Article5. Donjerković D, Zhang L, Scott DW. Regulation of p27Kip1 accumulation in murine B-lymphoma cells: role of c-Myc and calcium. Cell Growth Differ. 1999; 10:695–704.6. Chakraborti T, Das S, Mondal M, Roychoudhury S, Chakraborti S. Oxidant, mitochondria and calcium: an overview. Cell Signal. 1999; 11:77–85.7. Katz E, Deehan MR, Seatter S, Lord C, Sturrock RD, Harnett MM. B cell receptor-stimulated mitochondrial phospholipase A2 activation and resultant disruption of mitochondrial membrane potential correlate with the induction of apoptosis in WEHI-231 B cells. J Immunol. 2001; 166:137–147.8. van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000; 67:2–17.
Article9. Katz E, Lord C, Ford CA, Gauld SB, Carter NA, Harnett MM. Bcl-(xL) antagonism of BCR-coupled mitochondrial phospholipase A(2) signaling correlates with protection from apoptosis in WEHI-231 B cells. Blood. 2004; 103:168–176.
Article10. Lee HH, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc Natl Acad Sci USA. 1999; 96:9136–9141.11. Mineva ND, Rothstein TL, Meyers JA, Lerner A, Sonenshein GE. CD40 ligand-mediated activation of the de novo RelB NF-kappaB synthesis pathway in transformed B cells promotes rescue from apoptosis. J Biol Chem. 2007; 282:17475–17485.12. Watanabe K, Ichinose S, Hayashizaki K, Tsubata T. Induction of autophagy by B cell antigen receptor stimulation and its inhibition by costimulation. Biochem Biophys Res Commun. 2008; 374:274–281.
Article13. Yan BC, Adachi T, Tsubata T. ER stress is involved in B cell antigen receptor ligation-induced apoptosis. Biochem Biophys Res Commun. 2008; 365:143–148.
Article14. Lee JR. Reactive oxygen species play roles on B cell surface receptor CD40-mediated proximal and distal signaling events: effects of an antioxidant, N-acetyl-L-cysteine treatment. Mol Cell Biochem. 2003; 252:1–7.15. Tsien RY. New tetracarboxylate chelators for fluorescence measurement and photochemical manipulation of cytosolic free calcium concentrations. Soc Gen Physiol Ser. 1986; 40:327–345.16. Malgaroli A, Milani D, Meldolesi J, Pozzan T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987; 105:2145–2155.17. Nunez R. DNA measurement and cell cycle analysis by flow cytometry. Curr Issues Mol Biol. 2001; 3:67–70.18. Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006; 1:1458–1461.
Article19. Khan AA, Soloski MJ, Sharp AH, Schilling G, Sabatini DM, Li SH, Ross CA, Snyder SH. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996; 273:503–507.
Article20. Benschop RJ, Brandl E, Chan AC, Cambier JC. Unique signaling properties of B cell antigen receptor in mature and immature B cells: implications for tolerance and activation. J Immunol. 2001; 167:4172–4179.
Article21. Lee YJ, Kim NY, Suh YA, Lee C. Involvement of ROS in curcumin-induced autophagic cell death. Korean J Physiol Pharmacol. 2011; 15:1–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Signaling via Tumor Necrosis Factor-Associated Factors (TRAFs) by CD27 and CD40 in Mouse B Cells
- Role for CD40 and CD40L Expression in Generating CD8 T Cell Response to Minor Histcompatibility Antigen, H60
- Ca2+-dependent Long-term Inactivation of Cardiac Na+/Ca2+ Exchanger
- Expression of Ca2+-dependent Synaptotagmin Isoforms in Mouse and Rat Parotid Acinar Cells
- Role of Regulators of G-Protein Signaling 4 in Ca2+ Signaling in Mouse Pancreatic Acinar Cells