Korean J Physiol Pharmacol.
2011 Jun;15(3):171-177. 10.4196/kjpp.2011.15.3.171.
Myoplasmic [Ca2+], Crossbridge Phosphorylation and Latch in Rabbit Bladder Smooth Muscle
- Affiliations
-
- 1Department of Internal Medicine, Gangnung Asan Hospital, Gangnung 210-711, Korea.
- 2Department of Physiology, College of Medicine, Kwandong University, Gangnung 210-751, Korea. skwon2028@kd.ac.kr
- KMID: 1448921
- DOI: http://doi.org/10.4196/kjpp.2011.15.3.171
Abstract
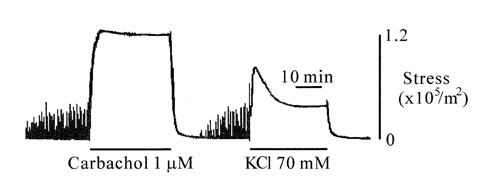
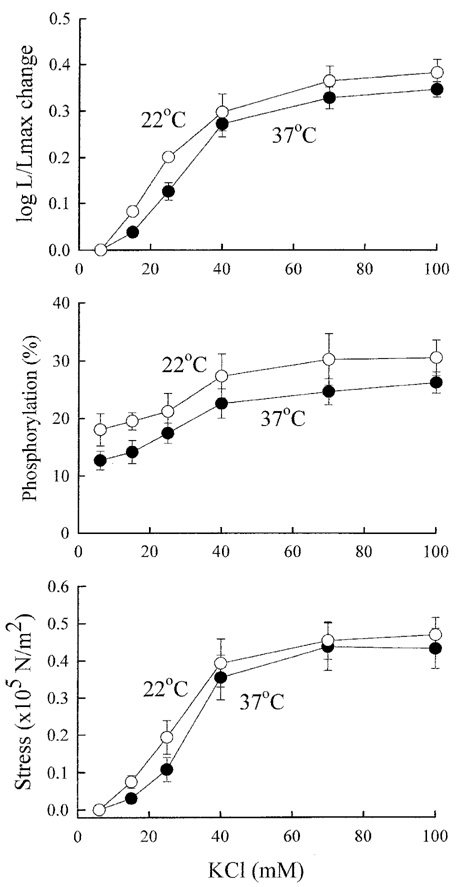
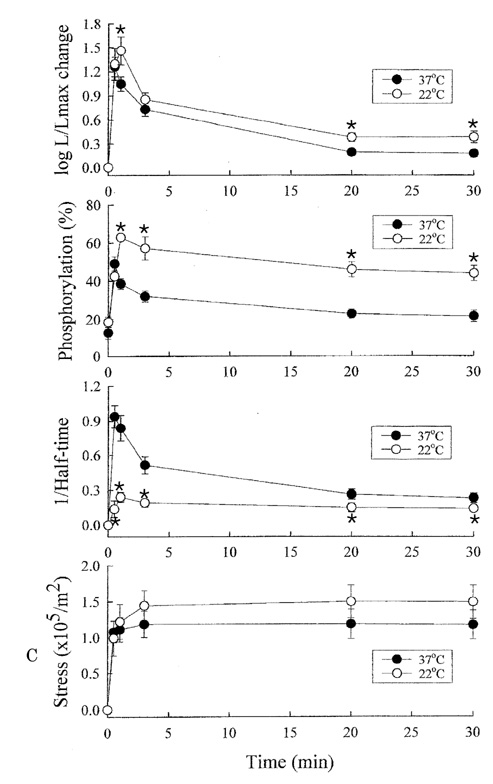
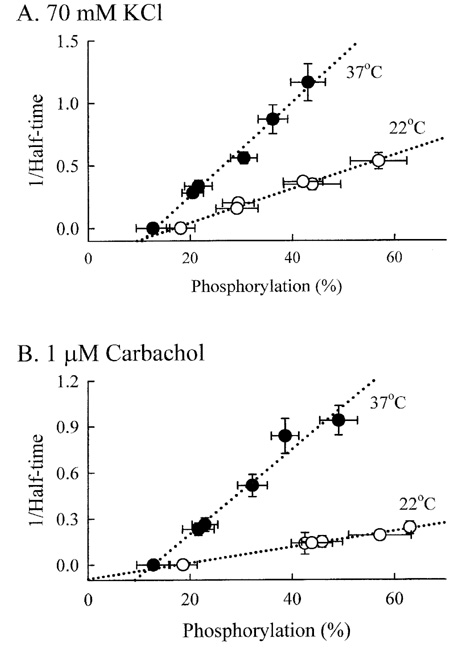
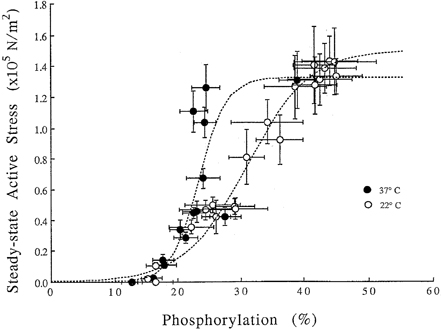
- Tonic smooth muscle exhibit the latch phenomenon: high force at low myosin regulatory light chains (MRLC) phosphorylation, shortening velocity (Vo), and energy consumption. However, the kinetics of MRLC phosphorylation and cellular activation in phasic smooth muscle are unknown. The present study was to determine whether Ca(2+)-stimulated MRLC phosphorylation could suffice to explain the agonist- or high K(+)-induced contraction in a fast, phasic smooth muscle. We measured myoplasmic [Ca2+], MRLC phosphorylation, half-time after step-shortening (a measure of Vo) and contractile stress in rabbit urinary bladder strips. High K(+)-induced contractions were phasic at both 22degrees C and 37degrees C: myoplasmic [Ca2+], MRLC phosphorylation, 1/half-time, and contractile stress increased transiently and then all decreased to intermediate values. Carbachol (CCh)-induced contractions exhibited latch at 37degrees C: stress was maintained at high levels despite decreasing myoplasmic [Ca2+], MRLC phosphorylation, and 1/half-time. At 22degrees C CCh induced sustained elevations in all parameters. 1/half-time depended on both myoplasmic [Ca2+] and MRLC phosphorylation. The steady-state dependence of stress on MRLC phosphorylation was very steep at 37degrees C in the CCh- or K(+)-depolarized tissue and reduced temperature flattend the dependence of stress on MRLC phosphorylation compared to 37degrees C. These data suggest that phasic smooth muscle also exhibits latch behavior and latch is less prominent at lower temperature.
Keyword
MeSH Terms
Figure
Reference
-
1. Murphy RA, Rembold CM. The latch-bridge hypothesis of smooth muscle contraction. Can J Physiol Pharmacol. 2005. 83:857–864.2. Somlyo AV, Somlyo AP. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968. 159:129–145.3. Hai CM, Murphy RA. Cross-bridge phosphorylation and regulation of latch state in smooth muscle. Am J Physiol. 1988. 254:C99–C106.4. Ogut O, Brozovich FV. Regulation of force in vascular smooth muscle. J Mol Cell Cardiol. 2003. 35:347–355.5. Hai CM, Murphy RA. Ca2+, crossbridge phosphorylation, and contraction. Annu Rev Physiol. 1989. 51:285–298.6. Hai CM, Murphy RA. Regulation of shortening velocity by cross-bridge phosphorylation in smooth muscle. Am J Physiol. 1988. 255:C86–C94.7. Gerthoffer WT, Murphy RA. Myosin phosphorylation and regulation of cross-bridge cycle in tracheal smooth muscle. Am J Physiol. 1983. 244:C182–C187.8. Rembold CM, Murphy RA. Latch-bridge model in smooth muscle: [Ca2+]i can quantitatively predict stress. Am J Physiol. 1990. 259:C251–C257.9. Rembold CM, Wardle RL, Wingard CJ, Batts TW, Etter EF, Murphy RA. Cooperative attachment of cross bridges predicts regulation of smooth muscle force by myosin phosphorylation. Am J Physiol Cell Physiol. 2004. 287:C594–C602.10. Aksoy MO, Murphy RA, Kamm KE. Role of Ca2+ and myosin light chain phosphorylation in regulation of smooth muscle. Am J Physiol. 1982. 242:C109–C116.11. Di Blasi P, Van Riper D, Kaiser R, Rembold CM, Murphy RA. Steady-state dependence of stress on cross-bridge phosphorylation in the swine carotid media. Am J Physiol. 1992. 262:C1388–C1391.12. Dillon PF, Aksoy MO, Driska SP, Murphy RA. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science. 1981. 211:495–497.13. Mitsui T, Kitazawa T, Ikebe M. Correlation between high temperature dependence of smooth muscle myosin light chain phosphatase activity and muscle relaxation rate. J Biol Chem. 1994. 269:5842–5848.14. Dillon PF, Murphy RA. Tonic force maintenance with reduced shortening velocity in arterial smooth muscle. Am J Physiol. 1982. 242:C102–C108.15. Rembold CM, Murphy RA. Myoplasmic [Ca2+] determines myosin phosphorylation in agonist-stimulated swine arterial smooth muscle. Circ Res. 1988. 63:593–603.16. Kamm KE, Stull JT. Myosin phosphorylation, force, and maximal shortening velocity in neurally stimulated tracheal smooth muscle. Am J Physiol. 1985. 249:C238–C247.17. Gong MC, Cohen P, Kitazawa T, Ikebe M, Masuo M, Somlyo AP, Somlyo AV. Myosin light chain phosphatase activities and the effects of phosphatase inhibitors in tonic and phasic smooth muscle. J Biol Chem. 1992. 267:14662–14668.18. Murphy RA, Aksoy MO, Dillon PF, Gerthoffer WT, Kamm KE. The role of myosin light chain phosphorylation in regulation of the cross-bridge cycle. Fed Proc. 1983. 42:51–56.19. Miller-Hance WC, Kamm KE. Force-velocity relation and myosin light chain phosphorylation in bovine coronary arterial smooth muscle. Circ Res. 1991. 69:1207–1214.20. Ozaki H, Gerthoffer WT, Publicover NG, Fusetani N, Sanders KM. Time-dependent changes in Ca2+ sensitivity during phasic contraction of canine antral smooth muscle. J Physiol. 1991. 440:207–224.21. Rembold CM, Murphy RA. Myoplasmic calcium, myosin phosphorylation, and regulation of the crossbridge cycle in swine arterial smooth muscle. Circ Res. 1986. 58:803–815.22. Horiuti K, Somlyo AV, Goldman YE, Somlyo AP. Kinetics of contraction initiated by flash photolysis of caged adenosine triphosphate in tonic and phasic smooth muscles. J Gen Physiol. 1989. 94:769–781.23. Blumenthal DK, Stull JT. Activation of skeletal muscle myosin light chain kinase by calcium(2+) and calmodulin. Biochemistry. 1980. 19:5608–5614.24. Pato MD, Tulloch AG, Walsh MP, Kerc E. Smooth muscle phosphatases: structure, regulation, and function. Can J Physiol Pharmacol. 1994. 72:1427–1433.25. Murphy RA. Arterial actomyosin: effects of pH and temperature on solubility and ATPase activity. Am J Physiol. 1971. 220:1494–1500.26. Lucius C, Arner A, Steusloff A, Troschka M, Hofmann F, Aktories K, Pfitzer G. Clostridium difficile toxin B inhibits carbachol-induced force and myosin light chain phosphorylation in guinea-pig smooth muscle: role of Rho proteins. J Physiol. 1998. 506:83–93.27. Wingard CJ, Nowocin JM, Murphy RA. Cross-bridge regulation by Ca(2+)-dependent phosphorylation in amphibian smooth muscle. Am J Physiol Regul Integr Comp Physiol. 2001. 281:R1769–R1777.28. Dillon PF, Murphy RA. High force development and crossbridge attachment in smooth muscle from swine carotid arteries. Circ Res. 1982. 50:799–804.29. Driska SP, Aksoy MO, Murphy RA. Myosin light chain phosphorylation associated with contraction in arterial smooth muscle. Am J Physiol. 1981. 240:C222–C233.30. Ratz PH, Hai CM, Murphy RA. Dependence of stress on cross-bridge phosphorylation in vascular smooth muscle. Am J Physiol. 1989. 256:C96–C100.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of caffeine on the Ca2+ pool affecting contractility and actomyosin ATPase activity in vascular smooth muscle of rabbit
- Effects of Vanadate and Pervanadate on Contraction of Cat Gastric Smooth Muscle
- Changes in intracellular Ca2+ concentration of rabbit coronary artery smooth muscle cell during ischemic cardioplegic period
- Effect of Pinacidil on the Contraction of Rabbit Carotid Artery
- Calcium Sensitization Mechanisms in Gastrointestinal Smooth Muscles