Anat Cell Biol.
2012 Mar;45(1):38-46. 10.5115/acb.2012.45.1.38.
Nestin expressing progenitor cells during establishment of the neural retina and its vasculature
- Affiliations
-
- 1Department of Anatomy, The Catholic University of Korea School of Medicine, Seoul, Korea. sujaoh@catholic.ac.kr
- KMID: 1447451
- DOI: http://doi.org/10.5115/acb.2012.45.1.38
Abstract
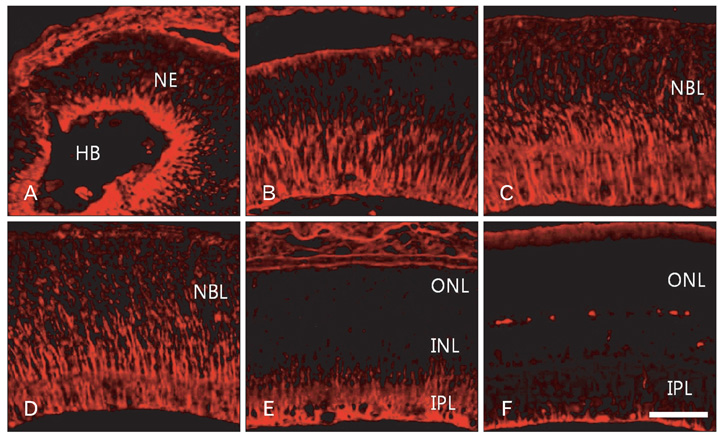
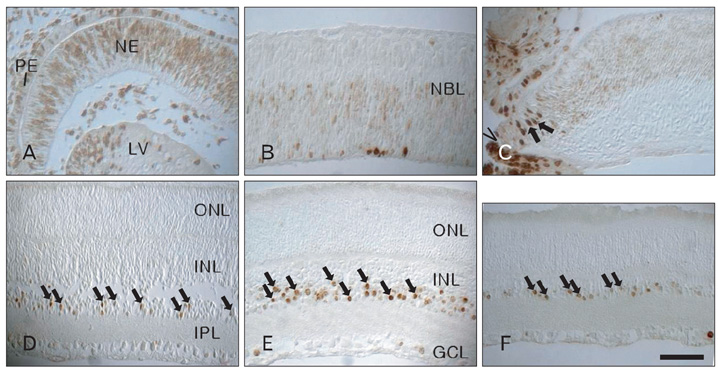
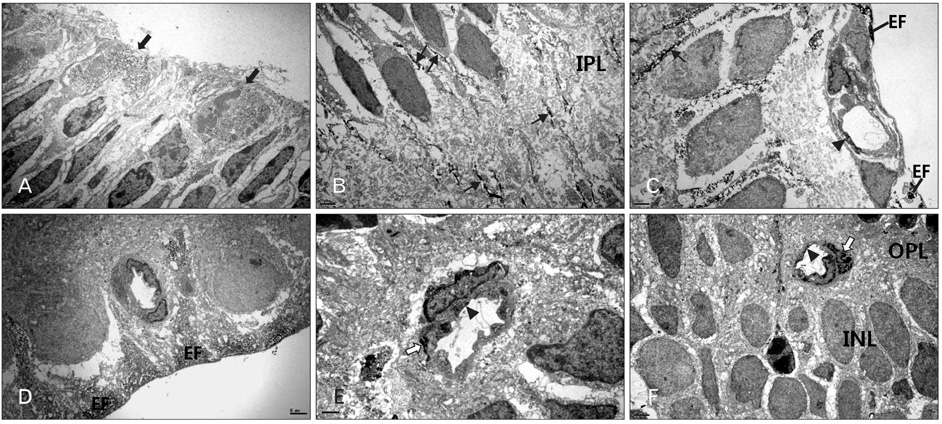
- In order to test if nestin is a useful marker for various types of progenitor cells, we explored nestin expression in the retina during development. Nestin expression was co-evaluated with bromodeoxyuridine (BrdU) labeling and Griffonia simplicifolia isolectin B4 (GSIB4) histochemistry. Nestin immunoreactivity appears in cell soma of dividing neural progenitor cells and their leading processes in retinas from embryonic day (E) 13 to E20, in accordance with a BrdU-labeled pattern. At postnatal day (P) 5, it is restricted to the end feet of Muller cells. BrdU-labeled nuclei were mainly in the inner part of the inner nuclear layer in postnatal neonates. The retinal vessels demarcated with GSIB4-positive endothelial cells were first distributed in the nerve fiber layer from P3. Afterward the vascular branches sprouted and penetrated deeply into the retina. The endothelial cells positive for GSIB4 and the pericytes in the microvessels were additionally immunoreactive for nestin. Interestingly, the presumed migrating microglial cells showing only GSIB4 reactivity preceded the microvessels throughout the neuroblast layer during vascular sprouting and extension. These findings may suggest that nestin expression represents the proliferation and movement potential of the neural progenitor cells as well as the progenitor cells of the endothelial cell and the pericyte during retinal development. Thus, Muller glial cells might be potential neural progenitor cells of the retina, and the retinal microvasculature established by both the endothelial and the pericyte progenitor cells via vasculogenesis along microglia migrating routes sustains its angiogenic potential.
Keyword
MeSH Terms
-
Bromodeoxyuridine
Carisoprodol
Endothelial Cells
Foot
Griffonia
Humans
Infant, Newborn
Intermediate Filament Proteins
Lectins
Microglia
Microvessels
Nerve Fibers
Nerve Tissue Proteins
Neurogenesis
Neuroglia
Pericytes
Plant Lectins
Retina
Retinal Vessels
Retinaldehyde
Stem Cells
Bromodeoxyuridine
Carisoprodol
Intermediate Filament Proteins
Lectins
Nerve Tissue Proteins
Plant Lectins
Retinaldehyde
Figure
Cited by 1 articles
-
Expression of Nestin on Endothelial Cells and Pericytes During Retinal Vascular Development in Mouse
Jin Soo Kim, Sung Wook Park, In Young Hwang, Yong Woo Kim, Jin Hyoung Kim, Jeong Hun Kim
J Korean Ophthalmol Soc. 2016;57(3):499-506. doi: 10.3341/jkos.2016.57.3.499.
Reference
-
1. Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993. 90:2074–2077.2. Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993. 11:173–189.3. Goldman SA, Zukhar A, Barami K, Mikawa T, Niedzwiecki D. Ependymal/subependymal zone cells of postnatal and adult songbird brain generate both neurons and nonneuronal siblings in vitro and in vivo. J Neurobiol. 1996. 30:505–520.4. Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994. 13:1071–1082.5. Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999. 97:703–716.6. Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999. 19:4462–4471.7. Xu H, Sta Iglesia DD, Kielczewski JL, Valenta DF, Pease ME, Zack DJ, Quigley HA. Characteristics of progenitor cells derived from adult ciliary body in mouse, rat, and human eyes. Invest Ophthalmol Vis Sci. 2007. 48:1674–1682.8. Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I. Neural stem cell properties of Müller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006. 299:283–302.9. Faillace MP, Julian D, Korenbrot JI. Mitotic activation of proliferative cells in the inner nuclear layer of the mature fish retina: regulatory signals and molecular markers. J Comp Neurol. 2002. 451:127–141.10. Fischer AJ. Neural regeneration in the chick retina. Prog Retin Eye Res. 2005. 24:161–182.11. Fischer AJ, Reh TA. Potential of Müller glia to become neurogenic retinal progenitor cells. Glia. 2003. 43:70–76.12. Marquardt T, Gruss P. Generating neuronal diversity in the retina: one for nearly all. Trends Neurosci. 2002. 25:32–38.13. Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004. 101:13654–13659.14. Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J Neurosci. 2007. 27:7028–7040.15. Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006. 25:397–424.16. Wu DM, Schneiderman T, Burgett J, Gokhale P, Barthel L, Raymond PA. Cones regenerate from retinal stem cells sequestered in the inner nuclear layer of adult goldfish retina. Invest Ophthalmol Vis Sci. 2001. 42:2115–2124.17. Otteson DC, Hitchcock PF. Stem cells in the teleost retina: persistent neurogenesis and injury-induced regeneration. Vision Res. 2003. 43:927–936.18. Yurco P, Cameron DA. Responses of Müller glia to retinal injury in adult zebrafish. Vision Res. 2005. 45:991–1002.19. Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000. 290:1779–1782.20. Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000. 290:1775–1779.21. Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000. 164:247–256.22. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.23. Hung SC, Cheng H, Pan CY, Tsai MJ, Kao LS, Ma HL. In vitro differentiation of size-sieved stem cells into electrically active neural cells. Stem Cells. 2002. 20:522–529.24. Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985. 5:3310–3328.25. Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990. 60:585–595.26. Bozanić D, Bocina I, Saraga-Babić M. Involvement of cytoskeletal proteins and growth factor receptors during development of the human eye. Anat Embryol (Berl). 2006. 211:367–377.27. Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, Temple S. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000. 28:69–80.28. Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001. 31:727–741.29. Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001. 409:714–720.30. Campbell K, Götz M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 2002. 25:235–238.31. Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001. 4:143–150.32. Marvin MJ, Dahlstrand J, Lendahl U, McKay RD. A rod end deletion in the intermediate filament protein nestin alters its subcellular localization in neuroepithelial cells of transgenic mice. J Cell Sci. 1998. 111(Pt 14):1951–1961.33. Eliasson C, Sahlgren C, Berthold CH, Stakeberg J, Celis JE, Betsholtz C, Eriksson JE, Pekny M. Intermediate filament protein partnership in astrocytes. J Biol Chem. 1999. 274:23996–24006.34. Steinert PM, Chou YH, Prahlad V, Parry DA, Marekov LN, Wu KC, Jang SI, Goldman RD. A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. Limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV alpha-internexin. J Biol Chem. 1999. 274:9881–9890.35. Herrmann H, Aebi U. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitec ture and cytodynamics. Curr Opin Cell Biol. 2000. 12:79–90.36. Chou YH, Khuon S, Herrmann H, Goldman RD. Nestin promotes the phosphorylation-dependent disassembly of vimentin intermediate filaments during mitosis. Mol Biol Cell. 2003. 14:1468–1478.37. Rapaport DH, Wong LL, Wood ED, Yasumura D, La Vail MM. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004. 474:304–324.38. Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RD, Trojanowski JQ. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992. 66:303–313.39. Mokrý J, Nemecek S. Angiogenesis of extra- and intraembryonic blood vessels is associated with expression of nestin in endothelial cells. Folia Biol (Praha). 1998. 44:155–161.40. Mokrý J, Nemecek S. Cerebral angiogenesis shows nestin expression in endothelial cells. Gen Physiol Biophys. 1999. 18:Suppl 1. 25–29.41. Gu H, Wang S, Messam CA, Yao Z. Distribution of nestin immunoreactivity in the normal adult human forebrain. Brain Res. 2002. 943:174–180.42. Sugawara K, Kurihara H, Negishi M, Saito N, Nakazato Y, Sasaki T, Takeuchi T. Nestin as a marker for proliferative endothelium in gliomas. Lab Invest. 2002. 82:345–351.43. Mokrý J, Cízková D, Filip S, Ehrmann J, Osterreicher J, Kolár Z, English D. Nestin expression by newly formed human blood vessels. Stem Cells Dev. 2004. 13:658–664.44. Chan-Ling T, Page MP, Gardiner T, Baxter L, Rosinova E, Hughes S. Desmin ensheathment ratio as an indicator of vessel stability: evidence in normal development and in retinopathy of prematurity. Am J Pathol. 2004. 165:1301–1313.45. Checchin D, Sennlaub F, Levavasseur E, Leduc M, Chemtob S. Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci. 2006. 47:3595–3602.46. Dyer MA, Cepko CL. Control of Müller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000. 3:873–880.47. Chang ML, Wu CH, Jiang-Shieh YF, Shieh JY, Wen CY. Reactive changes of retinal astrocytes and Muller glial cells in kainate-induced neuroexcitotoxicity. J Anat. 2007. 210:54–65.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Nestin on Endothelial Cells and Pericytes During Retinal Vascular Development in Mouse
- Distribution of the Immunoreactivity for Glycoprotein M6B in the Neurogenic Niche and Reactive Glia in the Injury Penumbra Following Traumatic Brain Injury in Mice
- Characterization of nestin expression in astrocytes in the rat hippocampal CA1 region following transient forebrain ischemia
- Expression Profiles of F4/80 and Nestin in Ocular Immune Cells Following Pharmaceutically Induced Retinal Degeneration in Adult Mice
- Interrelationships between the Retinal Neuroglia and Vasculature in Diabetes