Anat Cell Biol.
2012 Mar;45(1):1-16. 10.5115/acb.2012.45.1.1.
A review on gastric leptin: the exocrine secretion of a gastric hormone
- Affiliations
-
- 1Department of Pathology and Cell Biology, University of Montreal, Montreal, Quebec, Canada. moise.bendayan@umontreal.ca
- KMID: 1447448
- DOI: http://doi.org/10.5115/acb.2012.45.1.1
Abstract
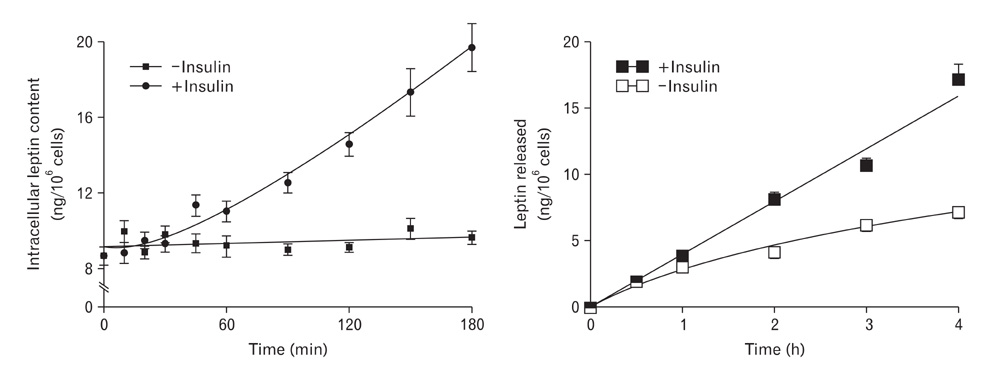
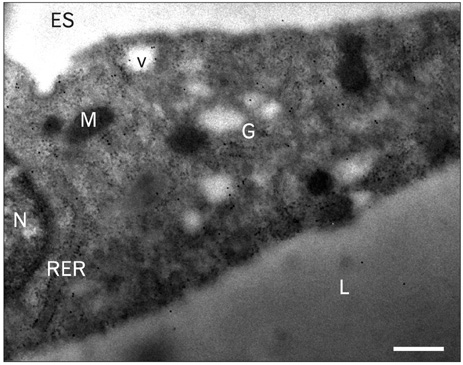
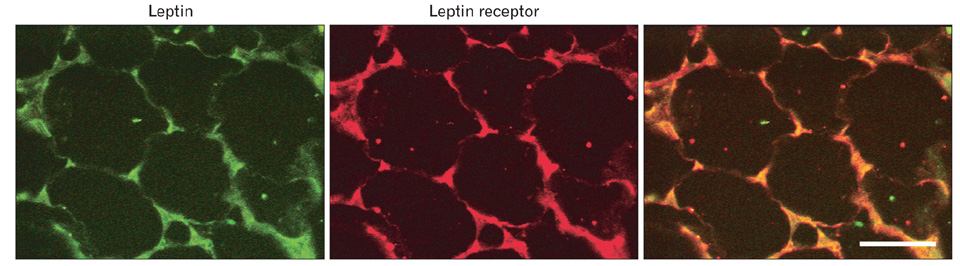
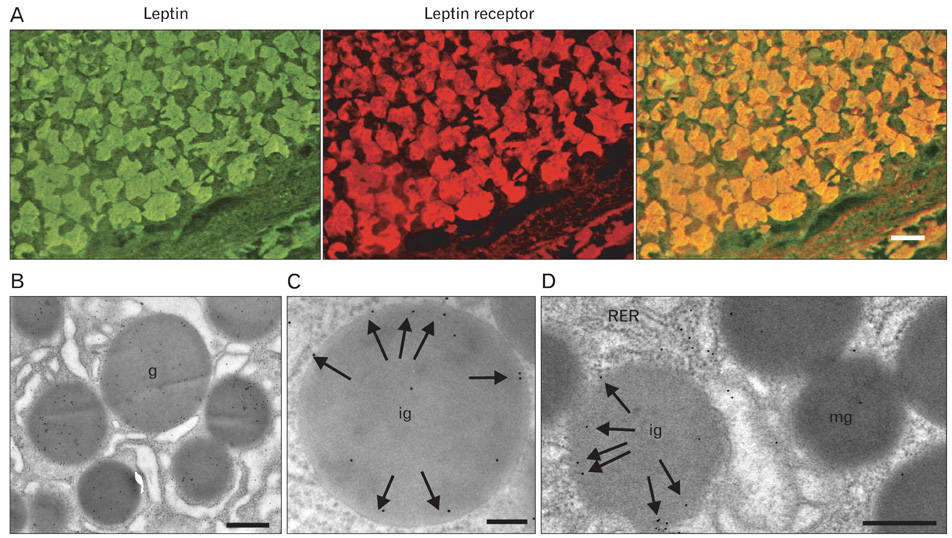
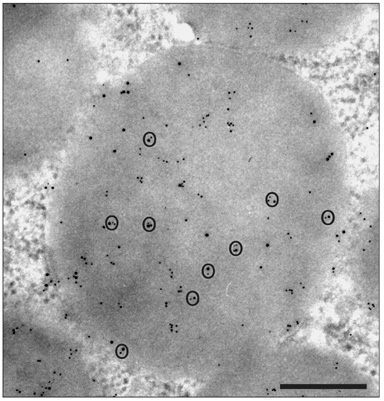
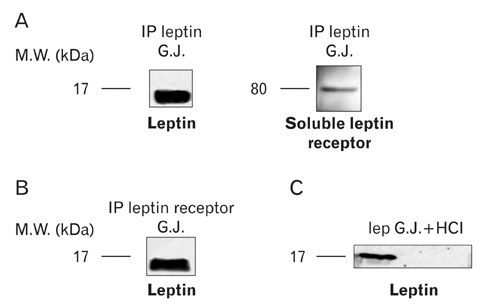
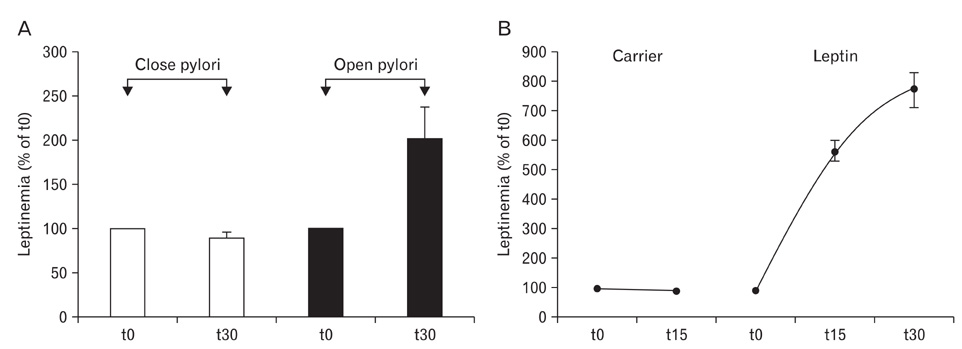
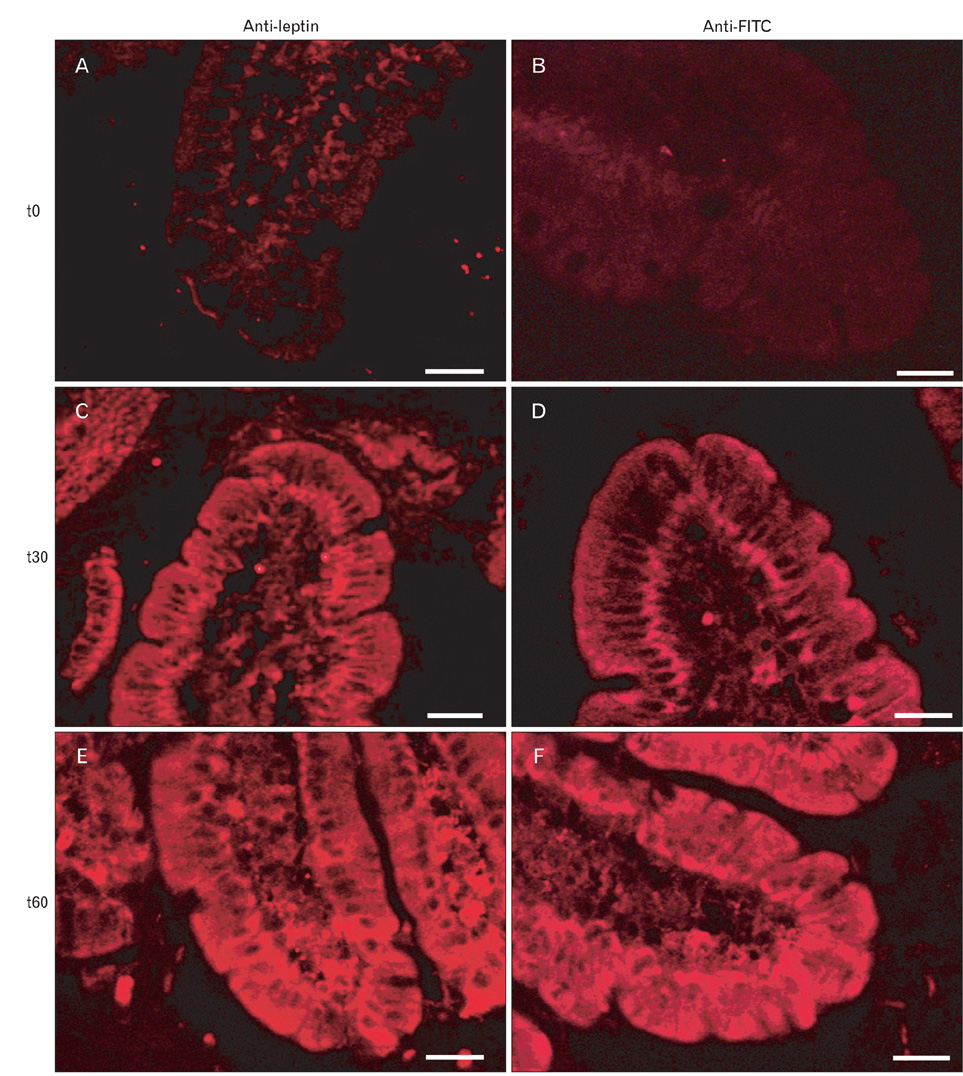
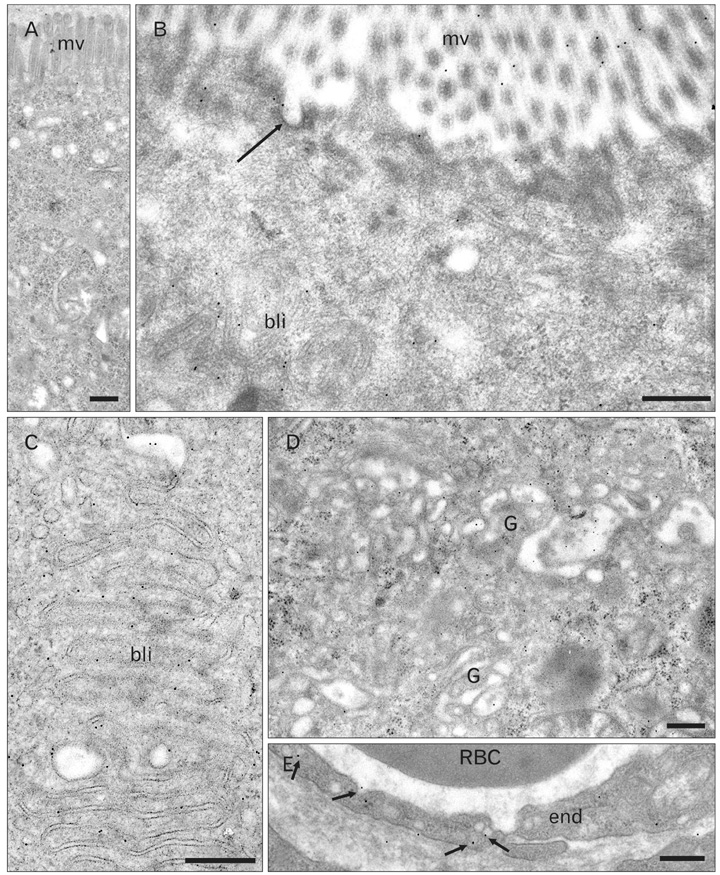
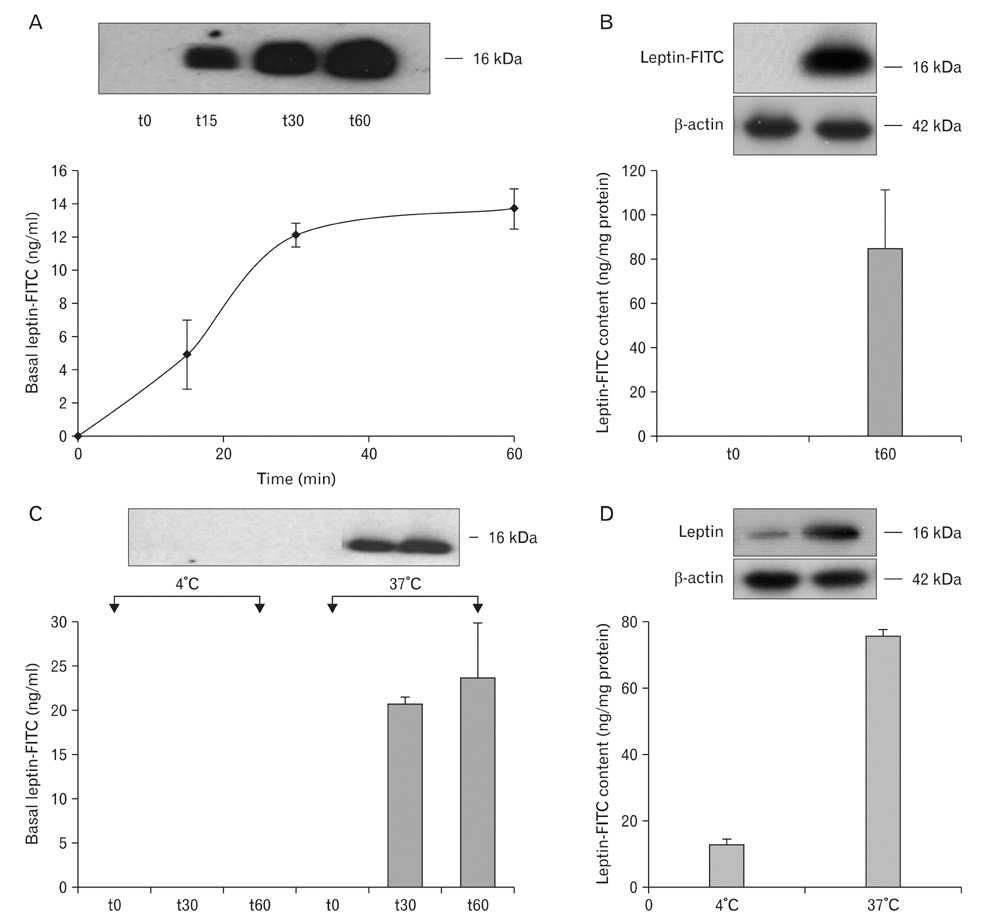
- A major advance in the understanding of the regulation of food intake has been the discovery of the adipokine leptin a hormone secreted by the adipose tissue. After crossing the blood-brain barrier, leptin reaches its main site of action at the level of the hypothalamic cells where it plays fundamental roles in the control of appetite and in the regulation of energy expenditure. At first considered as a hormone specific to the white adipose tissue, it was rapidly found to be expressed by other tissues. Among these, the gastric mucosa has been demonstrated to secrete large amounts of leptin. Secretion of leptin by the gastric chief cells was found to be an exocrine secretion. Leptin is secreted towards the gastric lumen into the gastric juice. We found that while secretion of leptin by the white adipose tissue is constitutive, secretion by the gastric cells is a regulated one responding very rapidly to secretory stimuli such as food intake. Exocrine-secreted leptin survives the hydrolytic conditions of the gastric juice by forming a complex with its soluble receptor. This soluble receptor is synthesized by the gastric cells and the leptin-leptin receptor complex gets formed at the level of the gastric chief cell secretory granules before being released into the gastric lumen. The leptin-leptin receptor upon resisting the hydrolytic conditions of the gastric juice is channelled, to the duodenum. Transmembrane leptin receptors expressed at the luminal membrane of the duodenal enterocytes interact with the luminal leptin. Leptin is actively transcytosed by the duodenal enterocytes. From the apical membrane it is transferred to the Golgi apparatus where it binds again its soluble receptor. The newly formed leptin-leptin receptor complex is then secreted baso-laterally into the intestinal mucosa to reach the blood capillaries and circulation thus reaching the hypothalamus where its action regulates food intake. Exocrine-secreted gastric leptin participates in the short term regulation of food intake independently from that secreted by the adipose tissue. Adipose tissue leptin on the other hand, regulates in the long term energy storage. Both tissues work in tandem to ensure management of food intake and energy expenditure.
MeSH Terms
-
Adipokines
Adipose Tissue
Adipose Tissue, White
Appetite
Blood-Brain Barrier
Capillaries
Chief Cells, Gastric
Dietary Sucrose
Duodenum
Eating
Energy Metabolism
Enterocytes
Gastric Juice
Gastric Mucosa
Golgi Apparatus
Hand
Hypothalamus
Intestinal Mucosa
Leptin
Membranes
Phenobarbital
Receptors, Leptin
Secretory Vesicles
Adipokines
Dietary Sucrose
Leptin
Phenobarbital
Receptors, Leptin
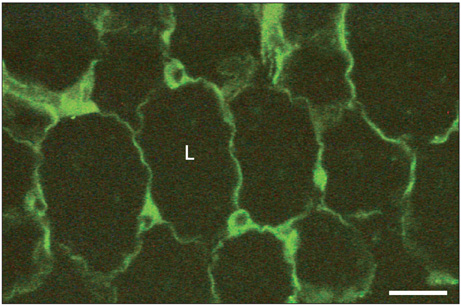
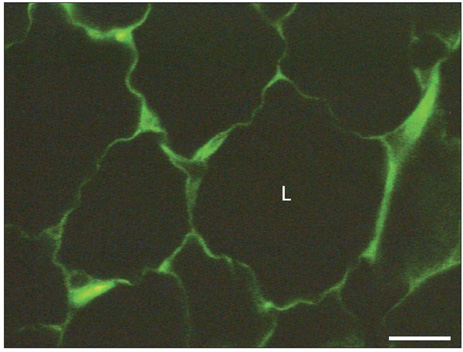
Figure
Reference
-
1. Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci. 1953. 140:578–596.2. Hervey GR. The effects of lesions in the hypothalamus in parabiotic rats. J Physiol. 1959. 145:336–352.3. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994. 372:425–432.4. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995. 83:1263–1271.5. Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996. 84:491–495.6. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996. 379:632–635.7. Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun. 2001. 283:982–988.8. Yang G, Ge H, Boucher A, Yu X, Li C. Modulation of direct leptin signaling by soluble leptin receptor. Mol Endocrinol. 2004. 18:1354–1362.9. Liebling DS, Eisner JD, Gibbs J, Smith GP. Intestinal satiety in rats. J Comp Physiol Psychol. 1975. 89:955–965.10. Guilmeau S, Buyse M, Tsocas A, Laigneau JP, Bado A. Duodenal leptin stimulates cholecystokinin secretion: evidence of a positive leptin-cholecystokinin feedback loop. Diabetes. 2003. 52:1664–1672.11. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007. 132:2131–2157.12. Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. 2008. 37:811–823.13. Unniappan S, Kieffer TJ. Leptin extends the anorectic effects of chronic PYY(3-36) administration in ad libitum-fed rats. Am J Physiol Regul Integr Comp Physiol. 2008. 295:R51–R58.14. Jeong KJ, Lee SY. High-level production of human leptin by fed-batch cultivation of recombinant Escherichia coli and its purification. Appl Environ Microbiol. 1999. 65:3027–3032.15. Zheng D, Jones JP, Usala SJ, Dohm GL. Differential expression of ob mRNA in rat adipose tissues in response to insulin. Biochem Biophys Res Commun. 1996. 218:434–437.16. Zheng D, Wooter MH, Zhou Q, Dohm GL. The effect of exercise on ob gene expression. Biochem Biophys Res Commun. 1996. 225:747–750.17. Cammisotto PG, Gingras D, Renaud C, Levy E, Bendayan M. Secretion of soluble leptin receptors by exocrine and endocrine cells of the gastric mucosa. Am J Physiol Gastrointest Liver Physiol. 2006. 290:G242–G249.18. Cammisotto PG, Bukowiecki LJ. Mechanisms of leptin secretion from white adipocytes. Am J Physiol Cell Physiol. 2002. 283:C244–C250.19. Cammisotto PG, Bukowiecki LJ, Deshaies Y, Bendayan M. Leptin biosynthetic pathway in white adipocytes. Biochem Cell Biol. 2006. 84:207–214.20. Cammisotto PG, Levy E, Bukowiecki LJ, Bendayan M. Crosstalk between adipose and gastric leptins for the control of food intake and energy metabolism. Prog Histochem Cytochem. 2010. 45:143–200.21. Cammisotto PG, Renaud C, Gingras D, Delvin E, Levy E, Bendayan M. Endocrine and exocrine secretion of leptin by the gastric mucosa. J Histochem Cytochem. 2005. 53:851–860.22. Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ. The stomach is a source of leptin. Nature. 1998. 394:790–793.23. Cinti S, Matteis RD, Picó C, Ceresi E, Obrador A, Maffeis C, Oliver J, Palou A. Secretory granules of endocrine and chief cells of human stomach mucosa contain leptin. Int J Obes Relat Metab Disord. 2000. 24:789–793.24. Lindqvist A, de la Cour CD, Stegmark A, Håkanson R, Erlanson-Albertsson C. Overeating of palatable food is associated with blunted leptin and ghrelin responses. Regul Pept. 2005. 130:123–132.25. Sánchez J, Oliver P, Palou A, Picó C. The inhibition of gastric ghrelin production by food intake in rats is dependent on the type of macronutrient. Endocrinology. 2004. 145:5049–5055.26. Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau JP, Attoub S, Lehy T, Henin D, Mignon M, Lewin MJ. Leptin secretion and leptin receptor in the human stomach. Gut. 2000. 47:178–183.27. Sobhani I, Buyse M, Goïot H, Weber N, Laigneau JP, Henin D, Soul JC, Bado A. Vagal stimulation rapidly increases leptin secretion in human stomach. Gastroenterology. 2002. 122:259–263.28. Morton NM, Emilsson V, Liu YL, Cawthorne MA. Leptin action in intestinal cells. J Biol Chem. 1998. 273:26194–26201.29. Breidert M, Miehlke S, Glasow A, Orban Z, Stolte M, Ehninger G, Bayerdörffer E, Nettesheim O, Halm U, Haidan A, Bornstein SR. Leptin and its receptor in normal human gastric mucosa and in Helicobacter pylori-associated gastritis. Scand J Gastroenterol. 1999. 34:954–961.30. Barrenetxe J, Villaro AC, Guembe L, Pascual I, Muñoz-Navas M, Barber A, Lostao MP. Distribution of the long leptin receptor isoform in brush border, basolateral membrane, and cytoplasm of enterocytes. Gut. 2002. 50:797–802.31. Cammisotto PG, Gingras D, Bendayan M. Transcytosis of gastric leptin through the rat duodenal mucosa. Am J Physiol Gastrointest Liver Physiol. 2007. 293:G773–G779.32. Schneeman BO. Gastrointestinal physiology and functions. Br J Nutr. 2002. 88:Suppl 2. S159–S163.33. Ducroc R, Guilmeau S, Akasbi K, Devaud H, Buyse M, Bado A. Luminal leptin induces rapid inhibition of active intestinal absorption of glucose mediated by sodium-glucose cotransporter 1. Diabetes. 2005. 54:348–354.34. El Homsi M, Ducroc R, Claustre J, Jourdan G, Gertler A, Estienne M, Bado A, Scoazec JY, Plaisancié P. Leptin modulates the expression of secreted and membrane-associated mucins in colonic epithelial cells by targeting PKC, PI3K, and MAPK pathways. Am J Physiol Gastrointest Liver Physiol. 2007. 293:G365–G373.35. Buyse M, Berlioz F, Guilmeau S, Tsocas A, Voisin T, Péranzi G, Merlin D, Laburthe M, Lewin MJ, Rozé C, Bado A. PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J Clin Invest. 2001. 108:1483–1494.36. Stan S, Levy E, Bendayan M, Zoltowska M, Lambert M, Michaud J, Asselin C, Delvin EE. Effect of human recombinant leptin on lipid handling by fully differentiated Caco-2 cells. FEBS Lett. 2001. 508:80–84.37. Igel M, Lindenthal B, Giesa U, von BK. Evidence that leptin contributes to intestinal cholesterol absorption in obese (ob/ob) mice and wild-type mice. Lipids. 2002. 37:153–157.38. Bendayan M, Ziv E, Ben-Sasson R, Bar-On H, Kidron M. Morpho-cytochemical and biochemical evidence for insulin absorption by the rat ileal epithelium. Diabetologia. 1990. 33:197–204.39. Bruneau N, Bendayan M, Gingras D, Ghitescu L, Levy E, Lombardo D. Circulating bile salt-dependent lipase originates from the pancreas via intestinal transcytosis. Gastroenterology. 2003. 124:470–480.40. Cloutier M, Gingras D, Bendayan M. Internalization and transcytosis of pancreatic enzymes by the intestinal mucosa. J Histochem Cytochem. 2006. 54:781–794.41. Cammisotto PG, Bendayan M, Sané A, Dominguez M, Garofalo C, Levy E. Receptor-mediated transcytosis of leptin through human intestinal cells in vitro. Int J Cell Biol. 2010. 2010:928169.42. Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995. 269:543–546.43. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995. 269:540–543.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Relevance of Plasma Leptin Levels to Dysmotility-Like Functional Dyspepsia
- Immunohistochemical Expression of Neuron Specific Enolase-Positive Cells in Gastric Adenocarcinomas
- Hypercalcemia as Initial Presentation of Metastatic Adenocarcinoma of Gastric Origin: A Case Report and Review of the Literature
- Ghrelin; Influences on Helicobacter pylori-associated Gastric Diseases
- Correlation between the Serum Leptin Level and the Expression of Leptin in Stomach Cancer Patients