Anat Cell Biol.
2011 Dec;44(4):295-303. 10.5115/acb.2011.44.4.295.
Different expressions of AQP1, AQP4, eNOS, and VEGF proteins in ischemic versus non-ischemic cerebropathy in rats: potential roles of AQP1 and eNOS in hydrocephalic and vasogenic edema formation
- Affiliations
-
- 1Department of Anatomy, Dongguk University College of Medicine, Gyeongju, Korea. jungyw@dongguk.ac.kr
- 2Section of Neuroscience Research, Medical Institute of Dongguk University, Gyeongju, Korea.
- KMID: 1447443
- DOI: http://doi.org/10.5115/acb.2011.44.4.295
Abstract
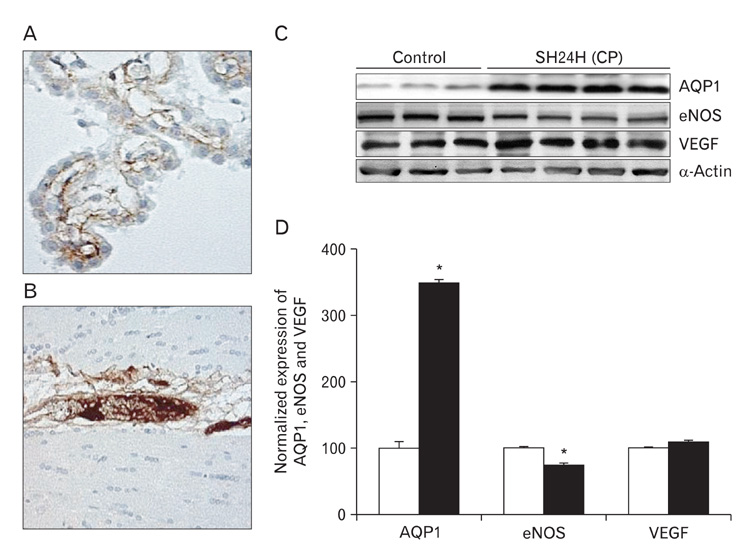
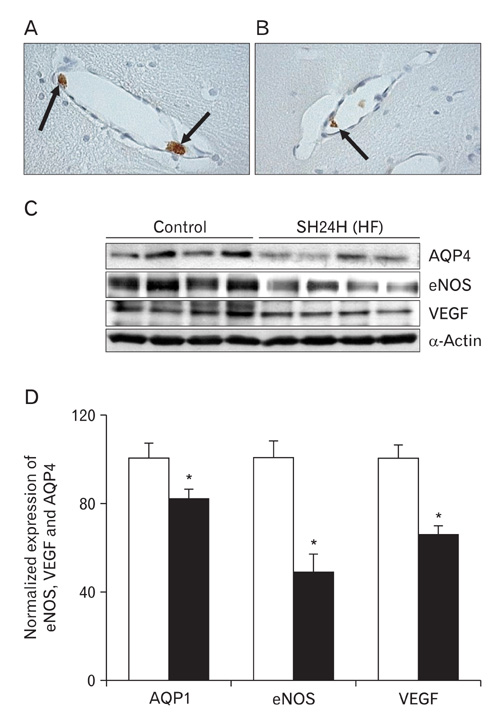
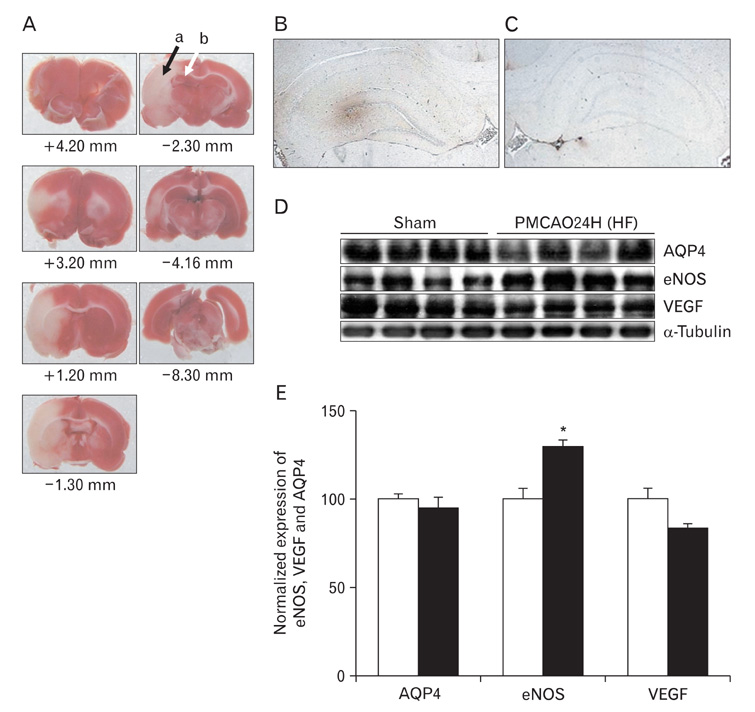
- In this study, expressions of aquaporin (AQP) 1, AQP4, endothelial nitric oxide synthase (eNOS), and vascular endothelial growth factor in blood-cerebrospinal fluid (CSF) barrier and blood-brain barrier (BBB) are examined in rat choroid plexus and peri-infarcted hippocampal formation (HF) following systemic hyponatremia (SH) and permanent middle cerebral artery occlusion (pMCAO). These events are thought to cause the development of hydrocephalic and vasogenic edemas. The importance of CSF overproduction and intact blood-CSF barrier during hydrocephalic edema formation is demonstrated by the high expression of AQP1 (329.86+/-10.2%, n=4 , P<0.01) and trapped plasma immunoglobulin G (IgG) in choroid plexus epithelium after 24 hours of SH. However, the increased eNOS expression in peri-infarcted HF (130+/-3%, n=4, P<0.01) and extravasation of plasma IgG into the extravascular compartment after 24 hours of pMCAO suggest that increased microvascular permeability, probably due to elevated levels of nitric oxide, leads to development of vasogenic brain edema via BBB breakdown. Based on these findings, the authors suggest that modulation of different protein expression, dependent on the type of brain edema, is required for primary (pMCAO) and secondary (SH) brain injuries to attenuate brain edema and neuronal degeneration.
Keyword
MeSH Terms
-
Animals
Aquaporin 1
Blood-Brain Barrier
Brain Edema
Brain Injuries
Capillary Permeability
Choroid Plexus
Edema
Epithelium
Hippocampus
Hyponatremia
Immunoglobulin G
Infarction, Middle Cerebral Artery
Neurons
Nitric Oxide
Nitric Oxide Synthase Type III
Plasma
Proteins
Rats
Vascular Endothelial Growth Factor A
Aquaporin 1
Immunoglobulin G
Nitric Oxide
Nitric Oxide Synthase Type III
Proteins
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Kimelberg HK. Current concepts of brain edema. Review of laboratory investigations. J Neurosurg. 1995. 83:1051–1059.2. Blei AT. Pathophysiology of brain edema in fulminant hepatic failure, revisited. Metab Brain Dis. 2001. 16:85–94.3. Jung YW, Choi IJ, Kwon TH. Altered expression of sodium transporters in ischemic penumbra after focal cerebral ischemia in rats. Neurosci Res. 2007. 59:152–159.4. Klatzo I. Evolution of brain edema concepts. Acta Neurochir Suppl (Wien). 1994. 60:3–6.5. Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004. 18:1291–1293.6. Spector R, Johanson CE. The mammalian choroid plexus. Sci Am. 1989. 261:68–74.7. Nielsen S, Smith BL, Christensen EI, Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A. 1993. 90:7275–7279.8. Oshio K, Song Y, Verkman AS, Manley GT. Aquaporin-1 deletion reduces osmotic water permeability and cerebrospinal fluid production. Acta Neurochir Suppl. 2003. 86:525–528.9. Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005. 19:76–78.10. Crone C. Modulation of solute permeability in microvascular endothelium. Fed Proc. 1986. 45:77–83.11. Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997. 17:171–180.12. Papadopoulos MC, Verkman AS. Aquaporin-4 and brain edema. Pediatr Nephrol. 2007. 22:778–784.13. Inoue H, Ando K, Wakisaka N, Matsuzaki K, Aihara M, Kumagai N. Effects of nitric oxide synthase inhibitors on vascular hyperpermeability with thermal injury in mice. Nitric Oxide. 2001. 5:334–342.14. Takeda M, Mori F, Yoshida A, Takamiya A, Nakagomi S, Sato E, Kiyama H. Constitutive nitric oxide synthase is associated with retinal vascular permeability in early diabetic rats. Diabetologia. 2001. 44:1043–1050.15. Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol. 1994. 267(3 Pt 1):L223–L241.16. Garcia JG, Schaphorst KL. Regulation of endothelial cell gap formation and paracellular permeability. J Investig Med. 1995. 43:117–126.17. Feng D, Nagy JA, Hipp J, Dvorak HF, Dvorak AM. Vesiculovacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J Exp Med. 1996. 183:1981–1986.18. Harrigan MR, Ennis SR, Masada T, Keep RF. Intraventricular infusion of vascular endothelial growth factor promotes cerebral angiogenesis with minimal brain edema. Neurosurgery. 2002. 50:589–598.19. Hasegawa H, Ma T, Skach W, Matthay MA, Verkman AS. Molecular cloning of a mercurial-insensitive water channel expre ssed in selected water-transporting tissues. J Biol Chem. 1994. 269:5497–5500.20. Praetorius J. Water and solute secretion by the choroid plexus. Pflugers Arch. 2007. 454:1–18.21. DiMattio J, Hochwald GM, Malhan C, Wald A. Effects of changes in serum osmolarity on bulk flow of fluid into cerebral ventricles and on brain water content. Pflugers Arch. 1975. 359:253–264.22. Bering EA Jr, Sato O. Hydrocephalus: changes in formation and absorption of cerebrospinal fluid within the cerebral ventricles. J Neurosurg. 1963. 20:1050–1063.23. Milhorat TH, Hammock MK, Fenstermacher JD, Levin VA. Cerebrospinal fluid production by the choroid plexus and brain. Science. 1971. 173:330–332.24. Tait MJ, Saadoun S, Bell BA, Papadopoulos MC. Water movements in the brain: role of aquaporins. Trends Neurosci. 2008. 31:37–43.25. Strange K. Regulation of solute and water balance and cell volume in the central nervous system. J Am Soc Nephrol. 1992. 3:12–27.26. Kubes P. Nitric oxide modulates epithelial permeability in the feline small intestine. Am J Physiol. 1992. 262(6 Pt 1):G1138–G1142.27. Janigro D, Leaman SM, Stanness KA. Dynamic modeling of the blood-brain barrier: a novel tool for studies of drug delivery to the brain. Pharm Sci Technolo Today. 1999. 2:7–12.28. Sivakumar V, Lu J, Ling EA, Kaur C. Vascular endothelial growth factor and nitric oxide production in response to hypoxia in the choroid plexus in neonatal brain. Brain Pathol. 2008. 18:71–85.29. Bloch O, Papadopoulos MC, Manley GT, Verkman AS. Aquaporin-4 gene deletion in mice increases focal edema associated with staphylococcal brain abscess. J Neurochem. 2005. 95:254–262.30. Kiening KL, van Landeghem FK, Schreiber S, Thomale UW, von Deimling A, Unterberg AW, Stover JF. Decreased hemispheric Aquaporin-4 is linked to evolving brain edema following controlled cortical impact injury in rats. Neurosci Lett. 2002. 324:105–108.31. Ke C, Poon WS, Ng HK, Lai FM, Tang NL, Pang JC. Impact of experimental acute hyponatremia on severe traumatic brain injury in rats: influences on injuries, permeability of blood-brain barrier, ultrastructural features, and aquaporin-4 expression. Exp Neurol. 2002. 178:194–206.32. Nag S, Picard P, Stewart DJ. Increased immunolocalization of nitric oxide synthases during blood-brain barrier breakdown and cerebral edema. Acta Neurochir Suppl. 2000. 76:65–68.33. Mayhan WG. Regulation of blood-brain barrier permeability. Microcirculation. 2001. 8:89–104.34. Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001. 42:2408–2413.35. van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, Tumas D, Gerlai R, Williams SP, van Lookeren Campagne M, Ferrara N. VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest. 1999. 104:1613–1620.36. Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002. 23:374–380.37. Elhusseiny A, Hamel E. Muscarinic--but not nicotinic--acetylcholine receptors mediate a nitric oxide-dependent dilation in brain cortical arterioles: a possible role for the M5 receptor subtype. J Cereb Blood Flow Metab. 2000. 20:298–305.38. Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998. 78:53–97.39. Zhang ZG, Reif D, Macdonald J, Tang WX, Kamp DK, Gentile RJ, Shakespeare WC, Murray RJ, Chopp M. ARL 17477, a potent and selective neuronal NOS inhibitor decreases infarct volume after transient middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1996. 16:599–604.40. Veltkamp R, Rajapakse N, Robins G, Puskar M, Shimizu K, Busija D. Transient focal ischemia increases endothelial nitric oxide synthase in cerebral blood vessels. Stroke. 2002. 33:2704–2710.41. Szpak GM, Lechowicz W, Lewandowska E, Bertrand E, Wierzba-Bobrowicz T, Dymecki J. Border zone neovascularization in cerebral ischemic infarct. Folia Neuropathol. 1999. 37:264–268.42. Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol. 2000. 156:965–976.43. Han F, Shirasaki Y, Fukunaga K. Microsphere embolism-induced endothelial nitric oxide synthase expression mediates disruption of the blood-brain barrier in rat brain. J Neurochem. 2006. 99:97–106.44. Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005. 39:51–70.45. Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007. 22:E4.46. Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002. 297:1186–1190.47. Melani A, Turchi D, Vannucchi MG, Cipriani S, Gianfriddo M, Pedata F. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int. 2005. 47:442–448.48. Windmüller O, Lindauer U, Foddis M, Einhäupl KM, Dirnagl U, Heinemann U, Dreier JP. Ion changes in spreading ischaemia induce rat middle cerebral artery constriction in the absence of NO. Brain. 2005. 128(Pt 9):2042–2051.49. Johanson CE, Palm DE, Primiano MJ, McMillan PN, Chan P, Knuckey NW, Stopa EG. Choroid plexus recovery after transient forebrain ischemia: role of growth factors and other repair mechanisms. Cell Mol Neurobiol. 2000. 20:197–216.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Different Expressions of eNOS and AQP4 after Focal Cerebral Ischemia in Rat: In Vasogenic Edema
- The Changes of AQP1 and AQP4 Expression after Focal Cerebral Ischemia in Rats
- Expression of Aquaporin-3 and Aquaporin-4 in Aquaporin-1 Knockout Mice
- The Expression of AQP1 and eNOS in Menopausal Rat Urinary Bladder
- Time-Dependent Expression Patterns of Cardiac Aquaporins Following Myocardial Infarction