Anat Cell Biol.
2011 Sep;44(3):194-203. 10.5115/acb.2011.44.3.194.
Resveratrol activates AMPK and suppresses LPS-induced NF-kappaB-dependent COX-2 activation in RAW 264.7 macrophage cells
- Affiliations
-
- 1Department of Anatomy and Neurobiology, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Korea. anaroh@gnu.ac.kr
- 2Department of Pharmacology, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Korea.
- 3Department of Thoracic and Cardiovascular Surgery, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Korea.
- KMID: 1447431
- DOI: http://doi.org/10.5115/acb.2011.44.3.194
Abstract
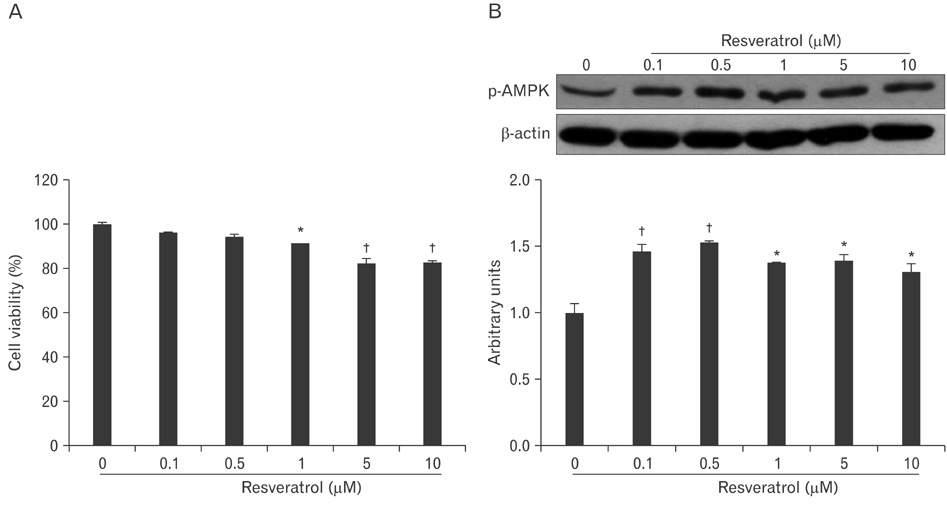
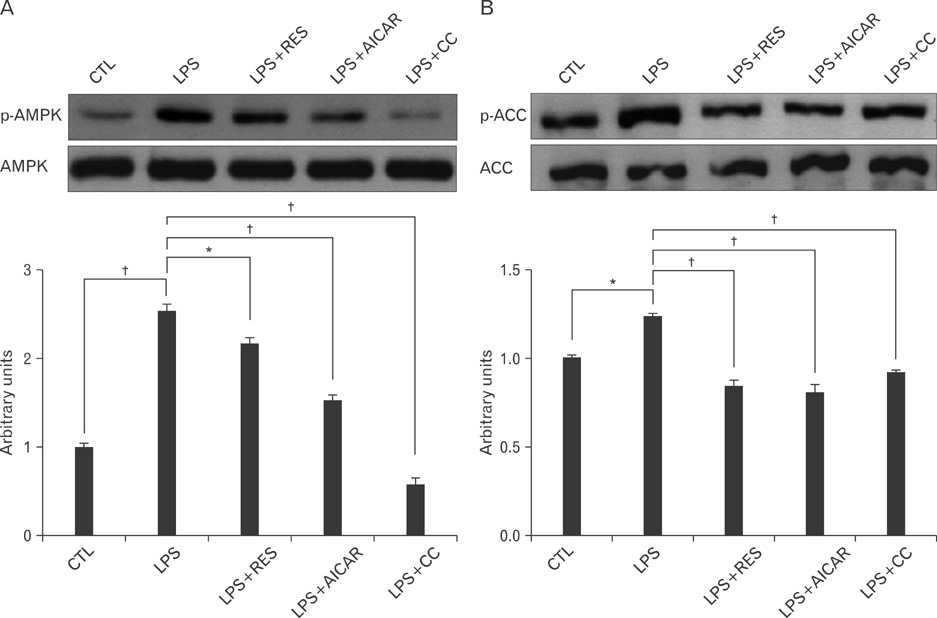
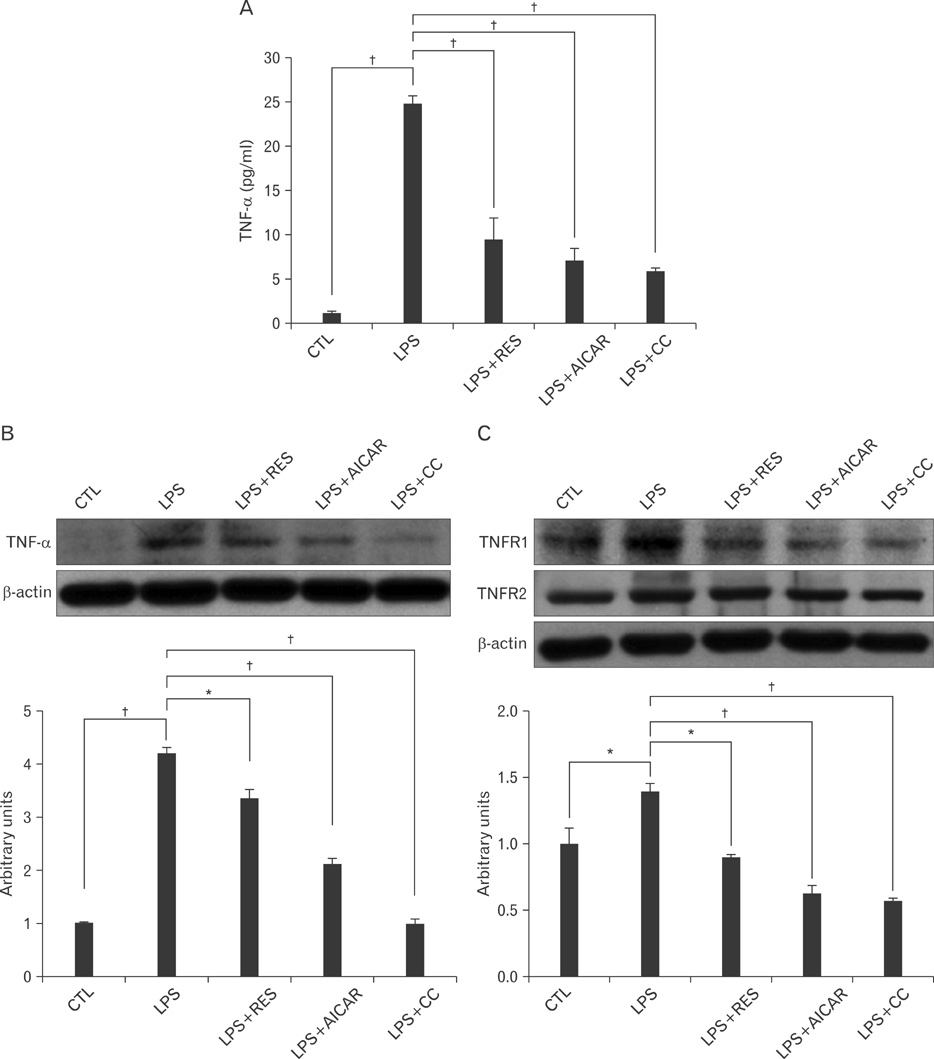
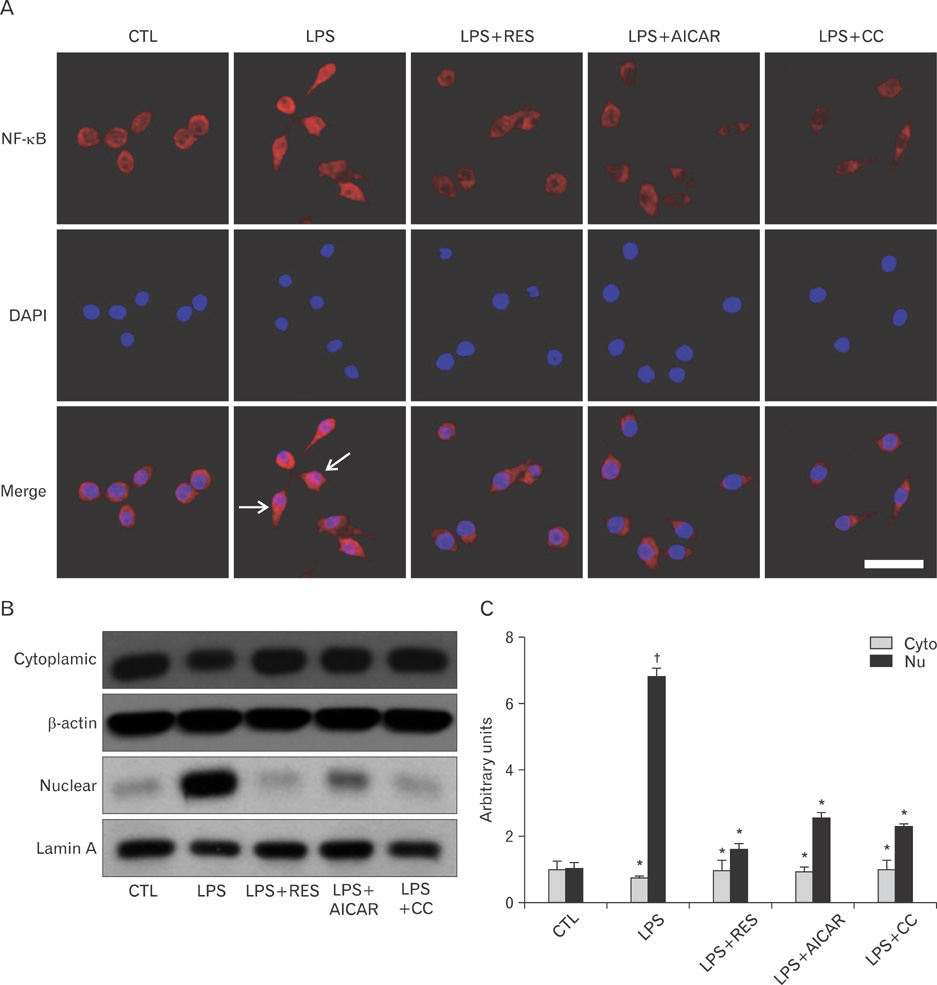
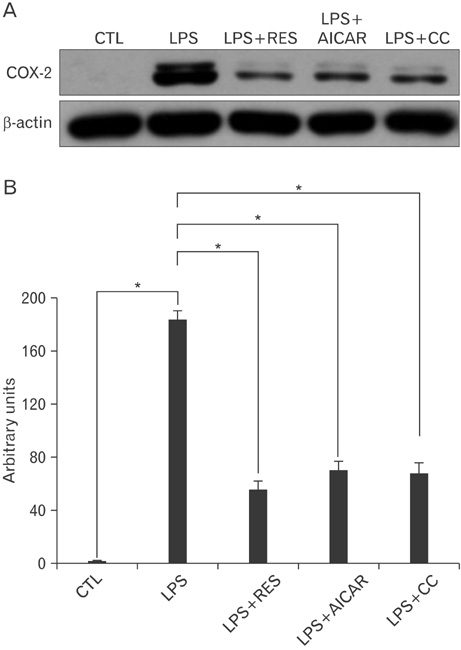
- AMP-activated protein kinase (AMPK), an enzyme involved in energy homeostasis, regulates inflammatory responses, but its precise mechanisms are not fully understood. Recent evidence has shown that resveratrol (RES), an AMPK activator, reduces prostaglandin E2 production in lipopolysaccharide (LPS)-treated microglia. Here, we examined the effect of RES on nuclear factor kappa B (NF-kappaB) dependent cyclooxygenase (COX)-2 activation in LPS-treated RWA 264.7 macrophages. We found that treatment with RES increased AMPK activation. AMPK and acetyl CoA carboxylase phosphorylation were attenuated in cells treated with LPS+RES, compared to cells treated with LPS alone. RES inhibited tumor necrosis factor (TNF)-alpha and TNF receptor 1 in LPS-treated cells. Finally, RES inhibited LPS-induced NF-kappaB translocation into the nucleus and COX-2 expression. Moreover, the effects of 5-aminoimidazole-4-carboxamide ribose and compound C were consistent with the effects of RES in LPS-treated cells. Taken together, these results suggest that the anti-inflammatory action of RES in RAW 264.7 macrophages is dependent on AMPK activation and is associated with inhibition of the LPS-stimulated NF-kappaB-dependent COX-2 signaling pathway.
MeSH Terms
-
Acetyl-CoA Carboxylase
AMP-Activated Protein Kinases
Dinoprostone
Homeostasis
Macrophages
Microglia
NF-kappa B
Phosphorylation
Prostaglandin-Endoperoxide Synthases
Receptors, Tumor Necrosis Factor
Ribose
Stilbenes
Tumor Necrosis Factor-alpha
AMP-Activated Protein Kinases
Acetyl-CoA Carboxylase
Dinoprostone
NF-kappa B
Prostaglandin-Endoperoxide Synthases
Receptors, Tumor Necrosis Factor
Ribose
Stilbenes
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006. 116:1776–1783.2. Corton JM, Gillespie JG, Hardie DG. Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol. 1994. 4:315–324.3. Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci. 1999. 24:22–25.4. Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002. 30(Pt 6):1064–1070.5. Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci. 2004. 24:479–487.6. Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem. 2002. 277:22131–22139.7. Raabe T, Bukrinsky M, Currie RA. Relative contribution of transcription and translation to the induction of tumor necrosis factor-alpha by lipopolysaccharide. J Biol Chem. 1998. 273:974–980.8. Wajant H, Scheurich P. Tumor necrosis factor receptor-associated factor (TRAF) 2 and its role in TNF signaling. Int J Biochem Cell Biol. 2001. 33:19–32.9. Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A. 1998. 95:570–575.10. Shishodia S, Potdar P, Gairola CG, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates cigarette smoke-induced NF-kappaB activation through inhibition of IkappaBalpha kinase in human lung epithelial cells: correlation with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis. 2003. 24:1269–1279.11. Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001. 2:149–156.12. Ermert L, Ermert M, Goppelt-Struebe M, Walmrath D, Grimminger F, Steudel W, Ghofrani HA, Homberger C, Duncker H, Seeger W. Cyclooxygenase isoenzyme localization and mRNA expression in rat lungs. Am J Respir Cell Mol Biol. 1998. 18:479–488.13. Frémont L. Biological effects of resveratrol. Life Sci. 2000. 66:663–673.14. Miller NJ, Rice-Evans CA. Antioxidant activity of resveratrol in red wine. Clin Chem. 1995. 41(12 Pt 1):1789.15. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006. 444:337–342.16. Kumar A, Sharma SS. NF-kappaB inhibitory action of resveratrol: a probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochem Biophys Res Commun. 2010. 394:360–365.17. Fullerton MD, Steinberg GR. SIRT1 takes a backseat to AMPK in the regulation of insulin sensitivity by resveratrol. Diabetes. 2010. 59:551–553.18. Müller JM, Ziegler-Heitbrock HW, Baeuerle PA. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology. 1993. 187:233–256.19. Arichi H, Kimura Y, Okuda H, Baba K, Kozawa M, Arichi S. Effects of stilbene components of the roots of Polygonum cuspidatum Sieb. et Zucc. on lipid metabolism. Chem Pharm Bull (Tokyo). 1982. 30:1766–1770.20. Sato M, Ray PS, Maulik G, Maulik N, Engelman RM, Bertelli AA, Bertelli A, Das DK. Myocardial protection with red wine extract. J Cardiovasc Pharmacol. 2000. 35:263–268.21. Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol. 1999. 126:673–680.22. Hardie DG. Regulation of fatty acid and cholesterol metabolism by the AMP-activated protein kinase. Biochim Biophys Acta. 1992. 1123:231–238.23. Pahan K, Sheikh FG, Namboodiri AM, Singh I. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest. 1997. 100:2671–2679.24. Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007. 10:1387–1394.25. Tamás P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, Cantrell DA. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006. 203:1665–1670.26. Blázquez C, Geelen MJ, Velasco G, Guzmán M. The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett. 2001. 489:149–153.27. McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005. 280:20493–20502.28. Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005. 25:9554–9575.29. Tracey KJ, Cerami A. Tumor necrosis factor: an updated review of its biology. Crit Care Med. 1993. 21:10 Suppl. S415–S422.30. Al-Lamki RS, Wang J, Skepper JN, Thiru S, Pober JS, Bradley JR. Expression of tumor necrosis factor receptors in normal kidney and rejecting renal transplants. Lab Invest. 2001. 81:1503–1515.31. Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008. 214:149–160.32. Jhun BS, Jin Q, Oh YT, Kim SS, Kong Y, Cho YH, Ha J, Baik HH, Kang I. 5-Aminoimidazole-4-carboxamide riboside suppresses lipopolysaccharide-induced TNF-alpha production through inhibition of phosphatidylinositol 3-kinase/Akt activation in RAW 264.7 murine macrophages. Biochem Biophys Res Commun. 2004. 318:372–380.33. Labuzek K, Liber S, Gabryel B, Bułdak L, Okopień B. Ambivalent effects of compound C (dorsomorphin) on inflammatory response in LPS-stimulated rat primary microglial cultures. Naunyn Schmiedebergs Arch Pharmacol. 2010. 381:41–57.34. Lu DY, Tang CH, Chen YH, Wei IH. Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J Cell Biochem. 2010. 110:697–705.35. Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol. 2009. 182:8005–8014.36. Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, Kim WS, Kim JB. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009. 296:E955–E964.37. Wang Y, Huang Y, Lam KS, Li Y, Wong WT, Ye H, Lau CW, Vanhoutte PM, Xu A. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc Res. 2009. 82:484–492.38. Zhou J, Zhou S, Tang J, Zhang K, Guang L, Huang Y, Xu Y, Ying Y, Zhang L, Li D. Protective effect of berberine on beta cells in streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Eur J Pharmacol. 2009. 606:262–268.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aromadendrin Inhibits Lipopolysaccharide-Induced Nuclear Translocation of NF-kappaB and Phosphorylation of JNK in RAW 264.7 Macrophage Cells
- The Regulatory Mechanism of Src Familly Kinase in Lipopolysaccharide (LPS) induced HF-kappaB Activation Pathway
- Rifampicin Inhibits the LPS-induced Expression of Toll-like Receptor 2 via the Suppression of NF-kappaB DNA-binding Activity in RAW 264.7 Cells
- Methyl p-Hydroxycinnamate Suppresses Lipopolysaccharide-Induced Inflammatory Responses through Akt Phosphorylation in RAW264.7 Cells
- Differential regulation of inducible nitric oxide synthase and cyclooxygenase-2 expression by superoxide dismutase in lipopolysaccharide stimulated RAW 264.7 cells