Anat Cell Biol.
2011 Sep;44(3):186-193. 10.5115/acb.2011.44.3.186.
Intra-renal slow cell-cycle cells contribute to the restoration of kidney tubules injured by ischemia/reperfusion
- Affiliations
-
- 1Department of Anatomy, Kyungpook National University School of Medicine, Daegu, Korea. kmpark@knu.ac.kr
- 2Department of Nursing, College of Nursing, Kyungpook National University, Daegu, Korea.
- KMID: 1447430
- DOI: http://doi.org/10.5115/acb.2011.44.3.186
Abstract
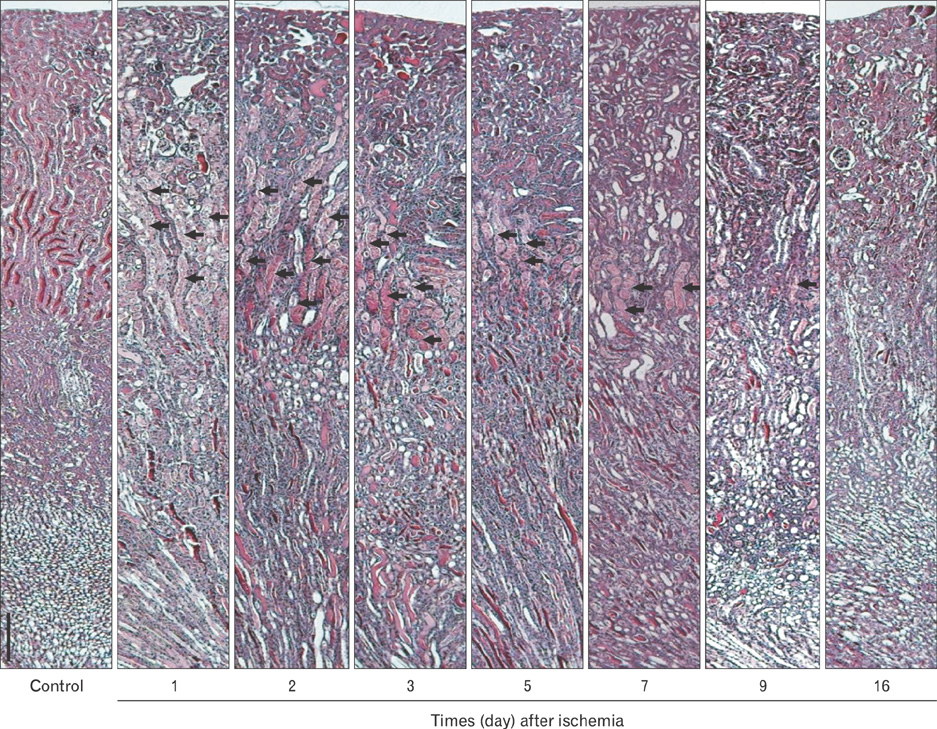
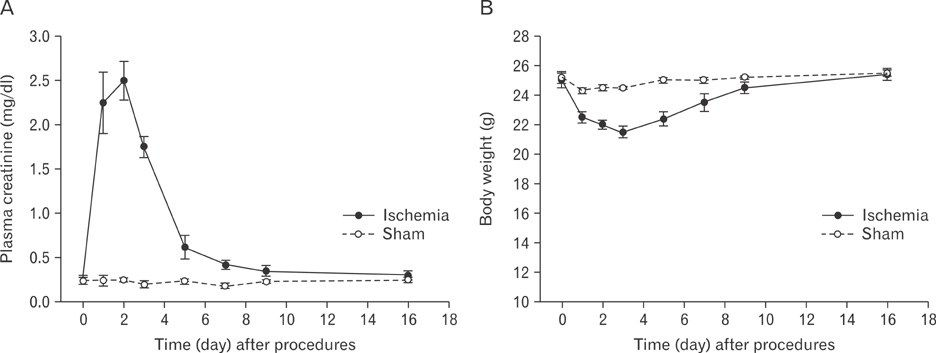
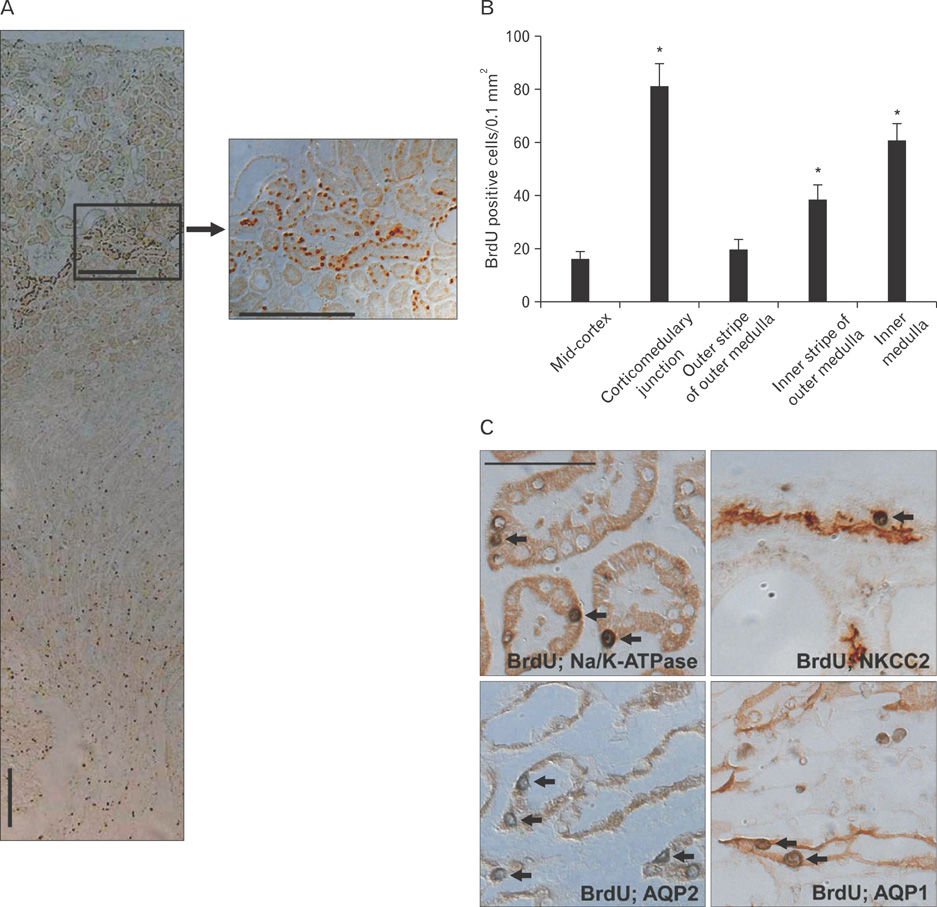
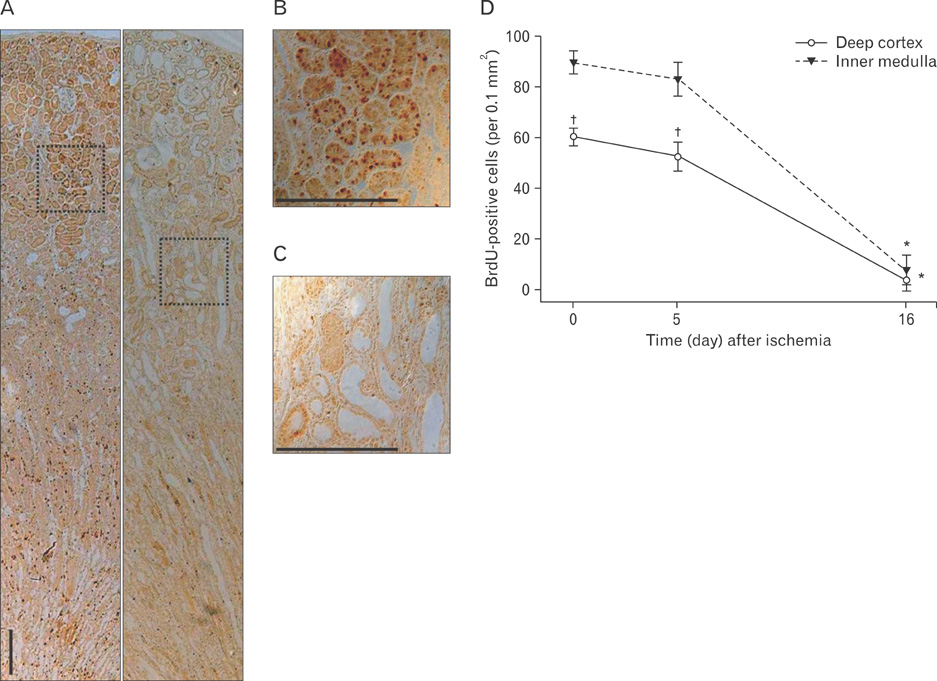
- Renal epithelial cells damaged by ischemia/reperfusion (I/R) can be restored by timely and appropriate treatment. Recent studies have reported that intra renal adult kidney stem cells contribute to the restoration of tubules damaged by I/R. Here, we determined the role of adult tubular cells in the restoration of damaged tubules. We labeled slow cell-cycle cells (SCCs) with 5-bromo-2'-deoxyuridine (BrdU) and investigated their location in the kidneys as well as their contribution to the restoration of tubular cells damaged by I/R injury in mice. Thirty minutes of bilateral ischemia resulted in severe disruption of tubular epithelial cells along with a decline in renal function. The post-ischemic disruption of tubular epithelial cells was most severe in the S3 segment of the outer stripe of the outer medulla. Damaged tubules demonstrated gradual recovery of renal function over time. BrdU-labeled SCCs were mainly observed in tubules located at the junction of cortex and outer medulla, as well as in the inner medulla. The tubular SCCs expressed functional tubule cell markers such as Na/K-ATPase, Na-K-Cl cotransporter-2, and aquaporin 1 and 2. BrdU-labeled SCCs survived I/R injury and proliferated. These results demonstrate that SCCs present in tubules contribute to the restoration of tubular epithelial cells injured by I/R.
MeSH Terms
Figure
Cited by 1 articles
-
Cyclosporin A aggravates hydrogen peroxide-induced cell death in kidney proximal tubule epithelial cells
Daeun Moon, Jinu Kim
Anat Cell Biol. 2019;52(3):312-323. doi: 10.5115/acb.18.192.
Reference
-
1. Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005. 68:1613–1617.2. Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996. 334:1448–1460.3. Brodie JC, Humes HD. Stem cell approaches for the treatment of renal failure. Pharmacol Rev. 2005. 57:299–313.4. Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005. 115:1743–1755.5. Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994. 93:2175–2188.6. Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int. 2002. 62:1285–1290.7. Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003. 112:42–49.8. Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, Igarashi P. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003. 14:1188–1199.9. Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001. 195:229–235.10. Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003. 14:Suppl 1. S55–S61.11. Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest. 2005. 115:1756–1764.12. Kim J, Park KM. Locations of slow-cycling cells, adult stem cells, in the organs of adult mouse. Korean J Anat. 2007. 40:347–357.13. Kim K, Lee KM, Han DJ, Yu E, Cho YM. Adult stem cell-like tubular cells reside in the corticomedullary junction of the kidney. Int J Clin Exp Pathol. 2008. 1:232–241.14. Kim K, Park BH, Ihm H, Kim KM, Jeong J, Chang JW, Cho YM. Expression of stem cell marker CD133 in fetal and adult human kidneys and pauci-immune crescentic glomerulonephritis. Histol Histopathol. 2011. 26:223–232.15. Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004. 114:795–804.16. Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci U S A. 2000. 97:13473–13475.17. Kim J, Kim JI, Kwon TH, Park KM. Kidney tubular cell regeneration starts in the deep cortex after ischemia. Korean J Nephrol. 2008. 27:536–544.18. Park KM, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem. 2001. 276:11870–11876.19. Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury, MAPK and MAPK kinase activation, and inflammation by remote transient ureteral obstruction. J Biol Chem. 2002. 277:2040–2049.20. Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005. 166:545–555.21. Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008. 2:284–291.22. Loverre A, Capobianco C, Ditonno P, Battaglia M, Grandaliano G, Schena FP. Increase of proliferating renal progenitor cells in acute tubular necrosis underlying delayed graft function. Transplantation. 2008. 85:1112–1119.23. Fujigaki Y, Goto T, Sakakima M, Fukasawa H, Miyaji T, Yamamoto T, Hishida A. Kinetics and characterization of initially regenerating proximal tubules in S3 segment in response to various degrees of acute tubular injury. Nephrol Dial Transplant. 2006. 21:41–50.24. Fujigaki Y, Sakakima M, Sun Y, Fujikura T, Tsuji T, Yasuda H, Hishida A. Cell division and phenotypic regression of proximal tubular cells in response to uranyl acetate insult in rats. Nephrol Dial Transplant. 2009. 24:2686–2692.25. Sakakima M, Fujigaki Y, Yamamoto T, Hishida A. A distinct population of tubular cells in the distal S3 segment contributes to S3 segment regeneration in rats following acute renal failure induced by uranyl acetate. Nephron Exp Nephrol. 2008. 109:e57–e70.26. Maeshima A, Yamashita S, Nojima Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol. 2003. 14:3138–3146.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Nestin in the Rat Kidney with Ischemia-Reperfusion Injury
- Th17 Cells Are Not Directly Associated with Renal Ischemia-Reperfusion Injury
- Mechanisms and therapeutic targets of ischemic acute kidney injury
- Kidney Tubular Cell Regeneration Starts in the Deep Cortex after Ischemia
- Does Heparin Attenuate the Renal Injury Induced by Ischemia Reperfusion in the Rabbit?