Lab Anim Res.
2011 Dec;27(4):327-331. 10.5625/lar.2011.27.4.327.
High glucose stimulates glutamate uptakes in pancreatic beta-cells
- Affiliations
-
- 1Department of Veterinary Physiology, College of Veterinary Medicine, Seoul National University, Seoul, Korea.
- 2Bio-therapy Human Resources Center, Animal Medical Center, Department of Veterinary Physiology, College of Veterinary Medicine, Chonnam National University, Gwangju, Korea. parksh@chonnam.ac.kr
- KMID: 1444965
- DOI: http://doi.org/10.5625/lar.2011.27.4.327
Abstract
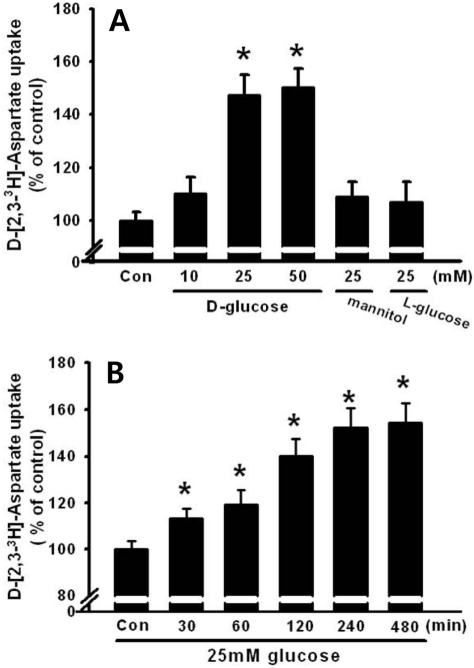
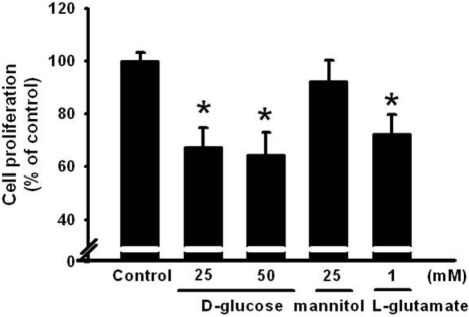
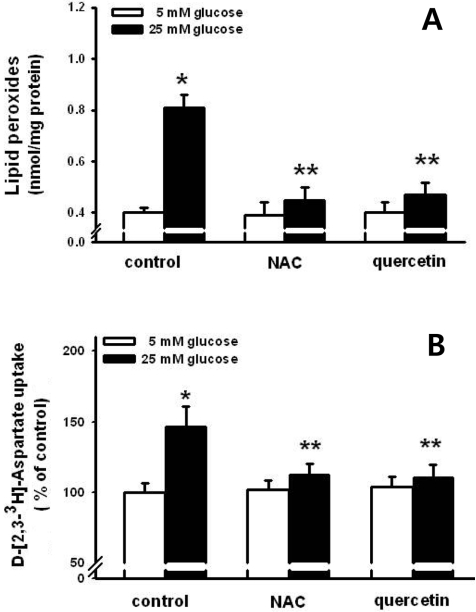
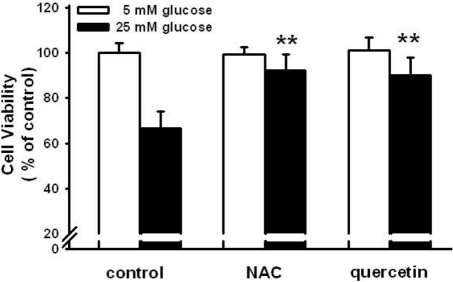
- Pancreatic beta-cells are major cells responsible for glucose metabolism in the body. Hyperglycemia is known to be a primary factor in the induction of diabetes mellitus. Glutamate is also an excitatory neurotransmitter in diverse organs. Oxidative stress also plays a pivotal role in the development of diabetes mellitus. However, the effect of hyperglycemia in glutamate uptake in the pancreas is not clear. Furthermore, the relationship between high glucose-induced glutamate uptake and oxidative stress has not been investigated. Therefore, this study was conducted to investigate the effect of high glucose on glutamate uptake in pancreatic beta-cells. In the present study, 25 mM glucose stimulated the glutamate uptake in HIT-15 cells of hamster pancreatic beta-cells. The treatment of 25 mM glucose and 1 mM glutamate also decreased the cell viability in HIT-15 cells. In addition, the treatment of 25 mM glucose induced an increase of lipid peroxide formation. High glucose-induced increase of LPO formation was prevented by the treatment of antioxidants such as N-acetyl-L-cysteine and quercetin. Furthermore, high glucose-induced stimulation of glutamate uptake and decrease of cell viability were also blocked by the treatment of N-acetyl-L-cysteine and quercetin. In conclusion, high glucose stimulated glutamate uptake via oxidative stress in pancreatic beta-cells.
MeSH Terms
Figure
Reference
-
1. Martens GA, Pipeleers D. Glucose, regulator of survival and phenotype of pancreatic beta cells. Vitam Horm. 2009; 80:507–539. PMID: 19251048.2. Thorens B. Glucose sensing and the pathogenesis of obesity and type 2 diabetes. Int J Obes (Lond). 2008; 32:S62–S71. PMID: 19079282.
Article3. Stumvoll M, Goldstein BJ, van Haeften TW. Pathogenesis of type 2 diabetes. Endocr Res. 2007; 32(1-2):19–37. PMID: 18271503.
Article4. Spooren W, Lesage A, Lavreysen H, Gasparini F, Steckler T. Metabotropic glutamate receptors: their therapeutic potential in anxiety. Curr Top Behav Neurosci. 2010; 2:391–413. PMID: 21309118.
Article5. Yasuo T, Kusuhara Y, Yasumatsu K, Ninomiya Y. Multiple receptor systems for glutamate detection in the taste organ. Biol Pharm Bull. 2008; 31(10):1833–1837. PMID: 18827337.
Article6. Morrison JF, Shehab S, Sheen R, Dhanasekaran S, Shaffiullah M, Mensah-Brown E. Sensory and autonomic nerve changes in the monosodium glutamate-treated rat: a model of type II diabetes. Exp Physiol. 2008; 93(2):213–222. PMID: 17911358.
Article7. Nawa A, Fujita-Hamabe W, Tokuyama S. Altered intestinal P-glycoprotein expression levels in a monosodium glutamateinduced obese mouse model. Life Sci. 2011; 89(23-24):834–838. PMID: 21983297.
Article8. Di Cairano ES, Davalli AM, Perego L, Sala S, Sacchi VF, La Rosa S, Finzi G, Placidi C, Capella C, Conti P, Centonze VE, Casiraghi F, Bertuzzi F, Folli F, Perego C. The glial glutamate transporter 1 (GLT1) is expressed by pancreatic β-cells and prevents glutamate-induced β-cell death. J Biol Chem. 2011; 286(16):14007–14018. PMID: 21335552.
Article9. Drews G, Krippeit-Drews P, Dufer M. Oxidative stress and beta-cell dysfunction. Pflugers Arch. 2010; 460(4):703–718. PMID: 20652307.
Article10. Stanton RC. Oxidative stress and diabetic kidney disease. Curr Diab Rep. 2011; 11(4):330–336. PMID: 21557044.
Article11. Whaley-Connell A, McCullough PA, Sowers JR. The role of oxidative stress in the metabolic syndrome. Rev Cardiovasc Med. 2011; 12(1):21–29. PMID: 21546885.12. Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic β-cells against glucose toxicity. Diabetes. 1999; 48(12):2398–2406. PMID: 10580429.13. Youl E, Bardy G, Magous R, Cros G, Sejalon F, Virsolvy A, Richard S, Quignard JF, Gross R, Petit P, Bataille D, Oiry C. Quercetin potentiates insulin secretion and protects INS-1 pancreatic β-cells against oxidative damage via the ERK1/2 pathway. Br J Pharmacol. 2010; 161(4):799–814. PMID: 20860660.
Article14. Wu F, Orlefors H, Bergström M, Antoni G, Omura H, Eriksson B, Watanabe Y, Långström B. Uptake of 14C- and 11C-labeled glutamate, glutamine and aspartate in vitro and in vivo. Anticancer Res. 2000; 20(1A):251–256. PMID: 10769663.15. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254. PMID: 942051.
Article16. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358. PMID: 36810.
Article17. Julio-Pieper M, Flor PJ, Dinan TG, Cryan JF. Exciting times beyond the brain: metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol Rev. 2011; 63(1):35–58. PMID: 21228260.
Article18. Cline GW, Zhao X, Jakowski AB, Soeller WC, Treadway JL. Islet-selectivity of G-protein coupled receptor ligands evaluated for PET imaging of pancreatic β-cell mass. Biochem Biophys Res Commun. 2011; 412(3):413–418. PMID: 21820405.
Article19. Hinoi E, Takarada T, Ueshima T, Tsuchihashi Y, Yoneda Y. Glutamate signaling in peripheral tissues. Eur J Biochem. 2004; 271(1):1–13. PMID: 14686914.
Article20. Di Cairano ES, Davalli AM, Perego L, Sala S, Sacchi VF, La Rosa S, Finzi G, Placidi C, Capella C, Conti P, Centonze VE, Casiraghi F, Bertuzzi F, Folli F, Perego C. The glial glutamate transporter 1 (GLT1) is expressed by pancreatic β-cells and prevents glutamate-induced β-cell death. J Biol Chem. 2011; 286(16):14007–14018. PMID: 21335552.
Article21. Bai L, Zhang X, Ghishan FK. Characterization of vesicular glutamate transporter in pancreatic alpha- and beta-cells and its regulation by glucose. Am J Physiol Gastrointest Liver Physiol. 2003; 284(5):G808–G814. PMID: 12444014.22. Gong L, Liu FQ, Wang J, Wang XP, Hou XG, Sun Y, Qin WD, Wei SJ, Zhang Y, Chen L, Zhang MX. Hyperglycemia induces apoptosis of pancreatic islet endothelial cells via reactive nitrogen species-mediated Jun N-terminal kinase activation. Biochim Biophys Acta. 2011; 1813(6):1211–1219. PMID: 21435358.23. Venieratos PD, Drossopoulou GI, Kapodistria KD, Tsilibary EC, Kitsiou PV. High glucose induces suppression of insulin signalling and apoptosis via upregulation of endogenous IL-1â and suppressor of cytokine signalling-1 in mouse pancreatic beta cells. Cell Signal. 2010; 22(5):791–800. PMID: 20067833.
Article24. Acharya JD, Ghaskadbi SS. Islets and their antioxidant defense. Islets. 2010; 2(4):225–235. PMID: 21099317.
Article25. Zhang Z, Liew CW, Handy DE, Zhang Y, Leopold JA, Hu J, Guo L, Kulkarni RN, Loscalzo J, Stanton RC. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and β-cell apoptosis. FASEB J. 2010; 24(5):1497–1505. PMID: 20032314.
Article26. Penugonda S, Mare S, Goldstein G, Banks WA, Ercal N. Effects of N-acetylcysteine amide (NACA), a novel thiol antioxidant against glutamate-induced cytotoxicity in neuronal cell line PC12. Brain Res. 2005; 1056(2):132–138. PMID: 16120436.27. Lim SK, Han HJ, Kim KY, Park SH. Both B1R and B2R act as intermediate signaling molecules in high glucose-induced stimulation of glutamate uptake in ARPE cells. J Cell Physiol. 2009; 221(3):677–687. PMID: 19725054.
Article28. Gaspar JM, Castilho A, Baptista FI, Liberal J, Ambrosio AF. Long-term exposure to high glucose induces changes in the content and distribution of some exocytotic proteins in cultured hippocampal neurons. Neuroscience. 2010; 171(4):981–992. PMID: 20950673.
Article29. Amonpatumrat S, Sakurai H, Wiriyasermkul P, Khunweeraphong N, Nagamori S, Tanaka H, Piyachaturawat P, Kanai Y. L-Glutamate enhances methylmercury toxicity by synergistically increasing oxidative stress. J Pharmacol Sci. 2008; 108(3):280–289. PMID: 19023177.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cause of Intracellular ATP dependency on Zn2++ Blockade of KATP Channels in Pancreatic Beta Cells
- Mechanistic insights into pancreatic beta-cell mass regulation by glucose and free fatty acids
- Oxidative Stress in Pancreatic Islet beta-cells Exposed to High Glucose Concentration
- Effect of Intracellular ATP on Zn2+ Blockade of KATP Channels in Pancreatic Beta Cells
- Beta-Cell Function and Nutrient Intake