Lab Anim Res.
2011 Dec;27(4):309-316. 10.5625/lar.2011.27.4.309.
Magnetic resonance imaging evaluation of Yukatan minipig brains for neurotherapy applications
- Affiliations
-
- 1Department of Veterinary Physiology, College of Veterinary Medicine, Chonnam National University, Gwangju, Korea.
- 2Department of Diagnostic Radiology, College of Medicine, Chosun University Hospital, Gwangju, Korea.
- 3College of Veterinary Medicine, Biotherapy Human Resources Center, Chonnam National University, Gwangju, Korea.
- 4Department of Veterinary Physiology, College of Veterinary Medicine and Research Institute for Veterinary Science, Seoul National University, Seoul, Korea. hjhan@snu.ac.kr
- KMID: 1444963
- DOI: http://doi.org/10.5625/lar.2011.27.4.309
Abstract
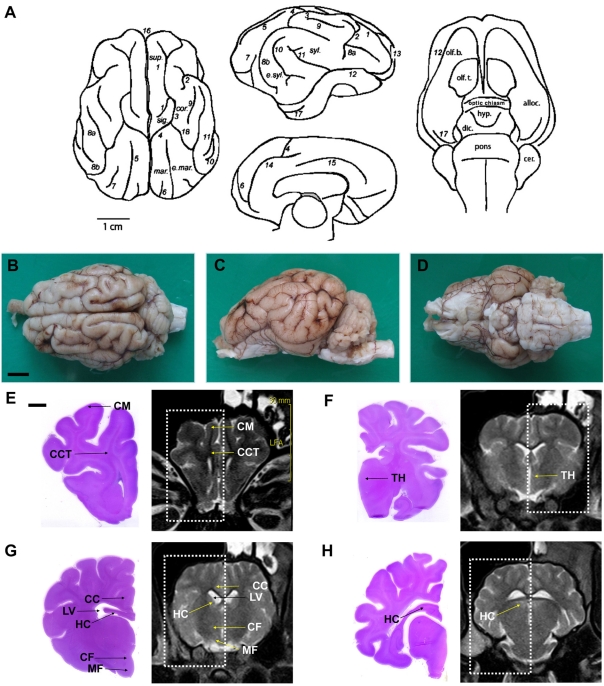
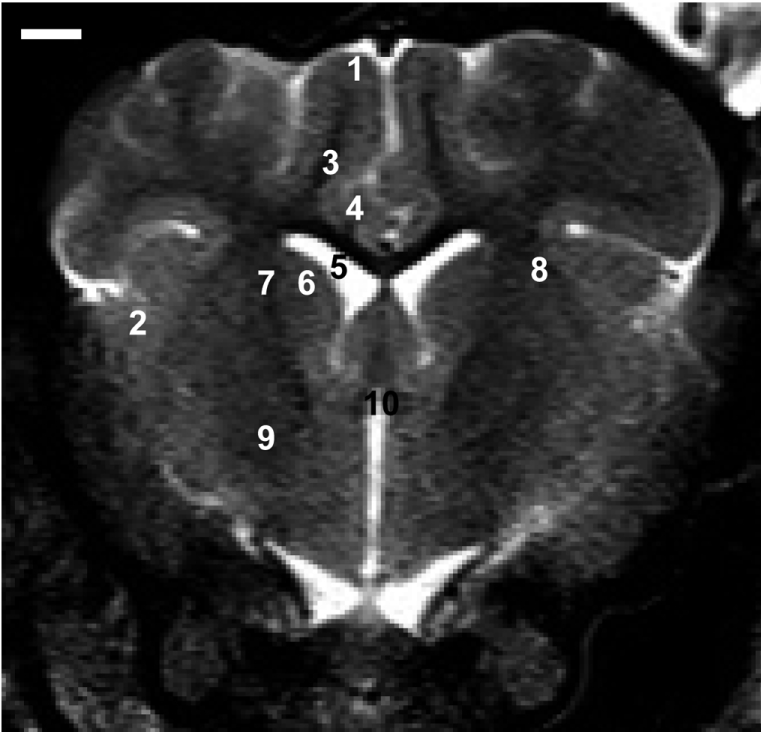
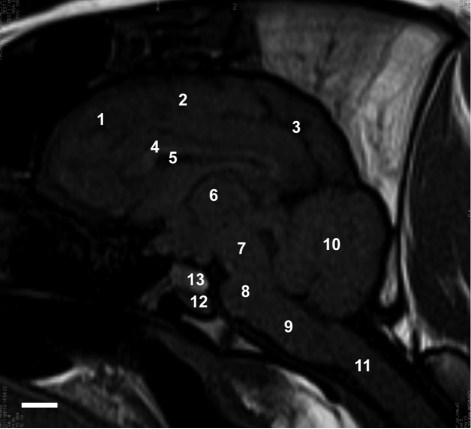
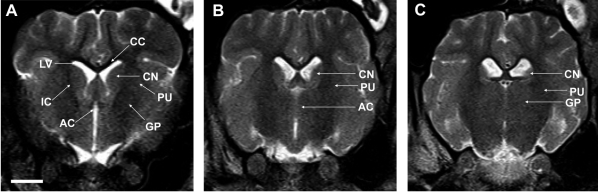
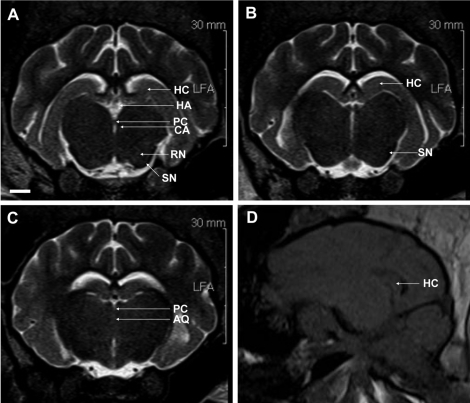
- Magnetic resonance imaging (MRI) of six Yukatan minipig brains was performed. The animals were placed in stereotaxic conditions currently used in experiments. To allow for correctpositioning of the animal in the MRI instrument, landmarks were previously traced on the snout of the pig. To avoid movements, animal were anesthetized. The animals were placed in a prone position in a Siemens Magnetom Avanto 1.5 System with a head coil. Axial T2-weighted and sagittal T1-weighted MRI images were obtained from each pig. Afterwards, the brains of the pigs were fixed and cut into axial sections. Histologic and MR images were compared. The usefulness of this technique is discussed.
MeSH Terms
Figure
Reference
-
1. Dooldeniya MD, Warrens AN. Xenotransplantation: Where are we today? J R Soc Med. 2003; 96(3):111–117. PMID: 12612110.
Article2. Lee KT, Byun MJ, Kang KS, Park EW, Lee SH, Cho S, Kim H, Kim KW, Lee T, Park JE, Park W, Shin D, Park HS, Jeon JT, Choi BH, Jang GW, Choi SH, Kim DW, Lim D, Park MR, Ott J, Schook LB, Kim TH. Neuronal genes for subcutaneous fat thickness in human and pig are identified by local genomic sequencing and combined SNP association study. PLoS One. 2011; 6(2):e16356. PMID: 21311593.
Article3. Hammer C. Xenotransplantation - will it bring the solution to organ shortage? Ann Transplant. 2004; 9(1):7–10. PMID: 15478879.4. Hannon JP, Bossone CA, Wade CE. Normal physiological values for conscious pigs used in biomedical research. Lab Anim Sci. 1990; 40(3):293–298. PMID: 2162986.5. Thors A, Muck P. Resorbable inferior vena cava filters: trial in an in-vivo porcine model. J Vasc Interv Radiol. 2011; 22(3):330–335. PMID: 21353986.
Article7. Ahn YK, Ryu JM, Jeong HC, Kim YH, Jeong MH, Lee MY, Lee SH, Park JH, Yun SP, Han HJ. Comparison of cardiac function and coronary angiography between conventional pigs and micropigs as measured by multidetector row computed tomography. J Vet Sci. 2008; 9(2):121–126. PMID: 18487932.
Article8. Andersen F, Watanabe H, Bjarkam C, Danielsen EH, Cumming P. Pig brain stereotaxic standard space: mapping of cerebral blood flow normative values and effect of MPTP-lesioning. Brain Res Bull. 2005; 66(1):17–29. PMID: 15925140.
Article9. Ho CS, Martens GW, Amoss MS Jr, Gomez-Raya L, Beattie CW, Smith DM. Swine leukocyte antigen (SLA) diversity in Sinclair and Hanford swine. Dev Comp Immunol. 2010; 34:250–257. PMID: 19782700.
Article10. Mezrich JD, Haller GW, Arn JS, Houser SL, Madsen JC, Sachs DH. Histocompatible miniature swine: an inbred large-animal model. Transplantation. 2003; 75(6):904–907. PMID: 12660524.11. Jelsing J, Gundersen HJ, Nielsen R, Hemmingsen R, Pakkenberg B. The postnatal development of cerebellar Purkinje cells in the Göttingen minipig estimated with a new stereological sampling technique--the vertical bar fractionator. J Anat. 2006; 209(3):321–331. PMID: 16928201.12. Lind NM, Moustgaard A, Jelsing J, Vajta G, Cumming P, Hansen AK. The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev. 2007; 31(5):728–751. PMID: 17445892.
Article13. Dullemeijer C, Zock PL, Coronel R, Den Ruijter HM, Katan MB, Brummer RJ, Kok FJ, Beekman J, Brouwer IA. Differences in fatty acid composition between cerebral brain lobes in juvenile pigs after fish oil feeding. Br J Nutr. 2008; 100(4):794–800. PMID: 18315890.
Article14. Smith DH, Cecil KM, Meaney DF, Chen XH, McIntosh TK, Gennarelli TA, Lenkinski RE. Magnetic resonance spectroscopy of diffuse brain trauma in the pig. J Neurotrauma. 1998; 15(9):665–674. PMID: 9753214.
Article15. Marcilloux JC, Félix MB, Rampin O, Stoffels C, Ibazizen MT, Cabanis EA, Laplace JP, Albe-Fessard D. Preliminary results of a magnetic resonance imaging (MRI) study of the pig brain placed in stereotaxic conditions. Neurosci Lett. 1993; 156(1-2):113–116. PMID: 8414170.
Article16. Rosendal F, Pedersen M, Sangill R, Stodkilde-Jorgensen H, Nielsen MS, Bjarkam CR, Sunde N, Sorensen JC. MRI protocol for in vivo visualization of the Göttingen minipig brain improves targeting in experimental functional neurosurgery. Brain Res Bull. 2009; 79(1):41–45. PMID: 19185604.
Article17. Danielsen EH, Cumming P, Andersen F, Bender D, Brevig T, Falborg L, Gee A, Gillings NM, Hansen SB, Hermansen F, Johansen J, Johansen TE, Dahl-Jørgensen A, Jorgensen HA, Meyer M, Munk O, Pedersen EB, Poulsen PH, Rodell AB, Sakoh M, Simonsen CZ, Smith DF, Sorensen JC, Ostergard L, Zimmer J, Gjedde A, Moller A. The DaNeX study of embryonic mesencephalic, dopaminergic tissue grafted to a minipig model of Parkinson's disease: preliminary findings of effect of MPTP poisoning on striatal dopaminergic markers. Cell Transplant. 2000; 9(2):247–259. PMID: 10811397.
Article18. Jelsing J, Olsen AK, Cumming P, Gjedde A, Hansen AK, Arnfred S, Hemmingsen R, Pakkenberg B. A volumetric screening procedure for the Göttingen minipig brain. Exp Brain Res. 2005; 162(4):428–435. PMID: 15668795.
Article19. Ruehe B, Niehues S, Heberer S, Nelson K. Miniature pigs as an animal model for implant research: bone regeneration in critical-size defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 108(5):699–706. PMID: 19782620.
Article20. Vodicka P, Smetana K Jr, Dvorankova B, Emerick T, Xu YZ, Ourednik J, Ourednik V, Motlik J. The miniature pig as an animal model in biomedical research. Ann N Y Acad Sci. 2005; 1049:161–171. PMID: 15965115.21. Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001; 2(4):280–291. PMID: 11283700.
Article22. Holm IE, West MJ. Hippocampus of the domestic pig: a stereological study of subdivisional volumes and neuron numbers. Hippocampus. 1994; 4(1):115–125. PMID: 8061750.
Article23. Dilberovic F, Secerov D, Tomic V. Morphological characteristics of the gyrus dentatus in some animal species and in man. Anat Anz. 1986; 161(3):231–238. PMID: 3717598.24. Holm IE, Geneser FA. Histochemical demonstration of zinc in the hippocampal region of the domestic pig: I. Entorhinal area, parasubiculum, and presubiculum. J Comp Neurol. 1989; 287(2):145–163. PMID: 2477401.
Article25. Felix B, Leger ME, Albe-Fessard D, Marcilloux JC, Rampin O, Laplace JP. Stereotaxic atlas of the pig brain. Brain Res Bull. 1999; 49(1-2):1–137. PMID: 10466025.26. Larsen MO, Rolin B. Use of the Göttingen minipig as a model of diabetes, with special focus on type 1 diabetes research. ILAR J. 2004; 45(3):303–313. PMID: 15229377.27. Freund E. Cytoarchitectonics of the mesencephalon and pons in the domestic pig (Susscrofa domestica). Anat Anz. 1969; 125(4):345–362. PMID: 4903679.28. Ostergaard K, Holm IE, Zimmer J. Tyrosine hydroxylase and acetylcholinesterase in the domestic pig mesencephalon: an immunocytochemical and histochemical study. J Comp Neurol. 1992; 322(2):149–166. PMID: 1355778.29. Dall AM, Danielsen EH, Sorensen JC, Andersen F, Moller A, Zimmer J, Gjedde AH, Cumming P. Quantitative [18F]fluorodopa/PET and histology of fetal mesencephalic dopaminergic grafts to the striatum of MPTP-poisoned minipigs. Cell Transplant. 2002; 11(8):733–746. PMID: 12588105.30. Smith PM, Blakemore WF. Porcine neural progenitors require commitment to the oligodendrocyte lineage prior to transplantation in order to achieve significant remyelination of demyelinated lesions in the adult CNS. Eur J Neurosci. 2000; 12(7):2414–2424. PMID: 10947820.
Article31. Bridge H, Clare S. High-resolution MRI: in vivo histology? Philos Trans R Soc Lond B Biol Sci. 2006; 361(1465):137–146. PMID: 16553313.32. Benabid AL. What the future holds for deep brain stimulation. Expert Rev Med Devices. 2007; 4(6):895–903. PMID: 18035954.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High field strength magnetic resonance imaging of cardiovascular diseases
- Deep Learning for Medical Image Analysis: Applications to Computed Tomography and Magnetic Resonance Imaging
- Efforts of the Past 20 Years for Proved Magnetic Resonance Imaging Safety of Medtronic Implantable Cardiac Devices
- Dynamic Contrast-Enhanced MRI and Its Applications in Various Central Nervous System Diseases
- Ultrafast Magnetic Resonance Imaging: Echo Planar Imaging and Spiral Scan Imaging