Tuberc Respir Dis.
2011 Feb;70(2):113-124. 10.4046/trd.2011.70.2.113.
The Macrophage-Specific Transcription Factor Can Be Modified Posttranslationally by Ubiquitination in the Lipopolysaccharide-Treated Macrophages
- Affiliations
-
- 1Divisioin of Allergy, Respiratory and Critical Care Medicine, Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea. basthma@cau.ac.kr
- 2Department of Pulmonary, Critical Care and Sleep Medicine, University of Illinois College of Medicine, Chicago, Illinois, USA.
- KMID: 1442860
- DOI: http://doi.org/10.4046/trd.2011.70.2.113
Abstract
- BACKGROUND
Macrophages are one of the most important inflammatory cells in innate immunity. PU.1 is a macrophage-specific transcription factor. Ubiquitins are the ultimate regulator of eukaryotic transcription. The ubiquitination process for PU.1 is unknown. This study investigated the lipopolysaccharide (LPS)-induced activation of PU.1 and its relation to ubiquitins in the macrophages.
METHODS
Raw264.7 cells, the primary cultured alveolar, pulmonary, and bone marrow derived macrophages were used. The Raw264.7 cells were treated with MG-132, NH4Cl, lactacytin and LPS. Nitric oxide and prostaglandin D2 and E2 were measured. Immunoprecipitation and Western blots were used to check ubiquitination of PU.1.
RESULTS
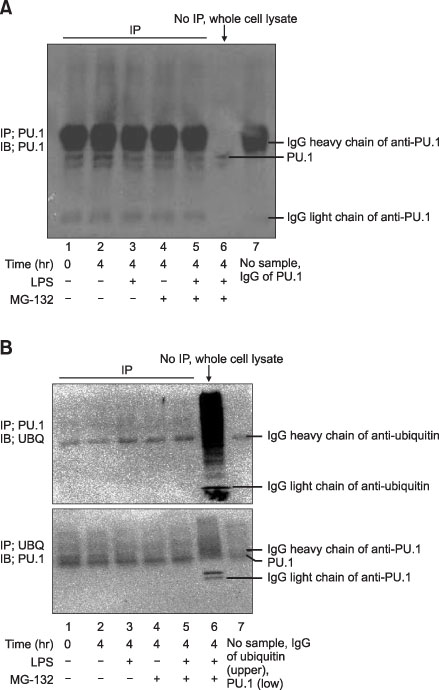
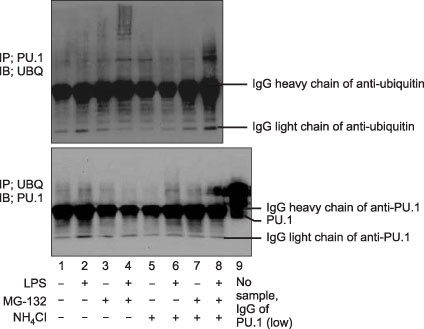
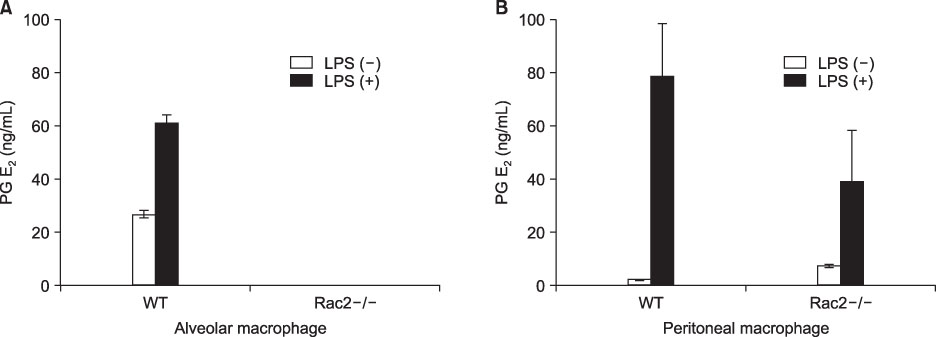
The PU.1 ubiquitination increased after LPS (1 microg/mL) treatment for 4 hours on Raw264.7 cells. The ubiquitination of PU.1 by LPS was increased by MG-132 or NH4Cl pretreatment. Two hours of LPS treatment on macrophages, PU.1 activation was not induced nor increased with the inhibition of proteasomes and/or lysosomes. The ubiquitination of PU.1 was increased in LPS-treated Raw264.7 cells at 12- and at 24 hours. LPS-treated cells increased nitric oxide production, which was diminished by MG-132 or NH4Cl. LPS increased the production of PGE2 in the alveolar and peritoneal macrophages of wild type mice; however, PGE2 was blocked or diminished in Rac2 null mice. Pretreatment of lactacystin increased PGE2, however it decreased the PGD2 level in the macrophages derived from the bone marrow of B57/BL6 mice.
CONCLUSION
LPS treatment in the macrophages ubiquitinates PU.1. Ubiquitination of PU.1 may be involved in synthesis of nitric oxide and prostaglandins.
Keyword
MeSH Terms
-
Acetylcysteine
Animals
Blotting, Western
Bone Marrow
Dinoprostone
Immunity, Innate
Immunoprecipitation
Leupeptins
Lysosomes
Macrophages
Macrophages, Peritoneal
Mice
Nitric Oxide
Prostaglandin D2
Prostaglandins
Proteasome Endopeptidase Complex
Transcription Factors
Ubiquitin
Ubiquitination
Ubiquitins
Acetylcysteine
Dinoprostone
Leupeptins
Nitric Oxide
Prostaglandin D2
Prostaglandins
Proteasome Endopeptidase Complex
Transcription Factors
Ubiquitin
Ubiquitins
Figure
Reference
-
1. Chopra M, Reuben JS, Sharma AC. Acute lung injury: apoptosis and signaling mechanisms. Exp Biol Med (Maywood). 2009. 234:361–371.2. Bellingan GJ. The pulmonary physician in critical care 6: the pathogenesis of ALI/ARDS. Thorax. 2002. 57:540–546.3. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008. 8:958–969.4. Joo M, Kwon M, Azim AC, Sadikot RT, Blackwell TS, Christman JW. Genetic determination of the role of PU.1 in macrophage gene expression. Biochem Biophys Res Commun. 2008. 372:97–102.5. Joo M, Kwon M, Cho YJ, Hu N, Pedchenko TV, Sadikot RT, et al. Lipopolysaccharide-dependent interaction between PU.1 and c-Jun determines production of lipocalin-type prostaglandin D synthase and prostaglandin D2 in macrophages. Am J Physiol Lung Cell Mol Physiol. 2009. 296:L771–L779.6. Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001. 15:557–567.7. Mazzi P, Donini M, Margotto D, Wientjes F, Dusi S. IFN-gamma induces gp91phox expression in human monocytes via protein kinase C-dependent phosphorylation of PU.1. J Immunol. 2004. 172:4941–4947.8. Lowenstein CJ, Alley EW, Raval P, Snowman AM, Snyder SH, Russell SW, et al. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci USA. 1993. 90:9730–9734.9. Hochstrasser M. Origin and function of ubiquitin-like proteins. Nature. 2009. 458:422–429.10. Sun F, Anantharam V, Zhang D, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Proteasome inhibitor MG-132 induces dopaminergic degeneration in cell culture and animal models. Neurotoxicology. 2006. 27:807–815.11. Barringhaus KG, Matsumura ME. The proteasome inhibitor lactacystin attenuates growth and migration of vascular smooth muscle cells and limits the response to arterial injury. Exp Clin Cardiol. 2007. 12:119–124.12. Hart PD, Young MR. Ammonium chloride, an inhibitor of phagosome-lysosome fusion in macrophages, concurrently induces phagosome-endosome fusion, and opens a novel pathway: studies of a pathogenic mycobacterium and a nonpathogenic yeast. J Exp Med. 1991. 174:881–889.13. Cao H, Xiao L, Park G, Wang X, Azim AC, Christman JW, et al. An improved LC-MS/MS method for the quantification of prostaglandins E2 and D2 production in biological fluids. Anal Biochem. 2008. 372:41–51.14. Zhang DE, Hetherington CJ, Chen HM, Tenen DG. The macrophage transcription factor PU.1 directs tissue-specific expression of the macrophage colony-stimulating factor receptor. Mol Cell Biol. 1994. 14:373–381.15. Tazawa R, Hamano E, Arai T, Ohta H, Ishimoto O, Uchida K, et al. Granulocyte-macrophage colony-stimulating factor and lung immunity in pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2005. 171:1142–1149.16. Rothenberg EV, Scripture-Adams DD. Competition and collaboration: GATA-3, PU.1, and Notch signaling in early T-cell fate determination. Semin Immunol. 2008. 20:236–246.17. Feldman AL, Arber DA, Pittaluga S, Martinez A, Burke JS, Raffeld M, et al. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone. Blood. 2008. 111:5433–5439.18. Marques C, Pereira P, Taylor A, Liang JN, Reddy VN, Szweda LI, et al. Ubiquitin-dependent lysosomal degradation of the HNE-modified proteins in lens epithelial cells. FASEB J. 2004. 18:1424–1426.19. Alonso S, Pethe K, Russell DG, Purdy GE. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc Natl Acad Sci USA. 2007. 104:6031–6036.20. Hirose S, Nishizumi H, Sakano H. Pub, a novel PU.1 binding protein, regulates the transcriptional activity of PU.1. Biochem Biophys Res Commun. 2003. 311:351–360.21. Bohuslav J, Chen LF, Kwon H, Mu Y, Greene WC. p53 induces NF-kappaB activation by an IkappaB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. J Biol Chem. 2004. 279:26115–26125.22. Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem. 2009. 78:363–397.23. Joo HY, Zhai L, Yang C, Nie S, Erdjument-Bromage H, Tempst P, et al. Regulation of cell cycle progression and gene expression by H2A deubiquitination. Nature. 2007. 449:1068–1072.24. Gammoh N, Gardiol D, Massimi P, Banks L. The Mdm2 ubiquitin ligase enhances transcriptional activity of human papillomavirus E2. J Virol. 2009. 83:1538–1543.25. Bachmaier K, Toya S, Gao X, Triantafillou T, Garrean S, Park GY, et al. E3 ubiquitin ligase Cblb regulates the acute inflammatory response underlying lung injury. Nat Med. 2007. 13:920–926.26. Yedgar S, Krimsky M, Cohen Y, Flower RJ. Treatment of inflammatory diseases by selective eicosanoid inhibition: a double-edged sword? Trends Pharmacol Sci. 2007. 28:459–464.27. Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J Exp Med. 2009. 206:61–68.28. Song L, Bhattacharya S, Yunus AA, Lima CD, Schindler C. Stat1 and SUMO modification. Blood. 2006. 108:3237–3244.29. Tillmanns S, Otto C, Jaffray E, Du Roure C, Bakri Y, Vanhille L, et al. SUMO modification regulates MafBdriven macrophage differentiation by enabling Myb-dependent transcriptional repression. Mol Cell Biol. 2007. 27:5554–5564.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of NFATc1 in Osteoclast Differentiation
- Regulation of Macrophage Activation and Differentiation in Atherosclerosis
- Macrophage Polarization and Infection
- Immunological Activities of Korean Mistletoe Extract ( Viscum album coloratum ; KM - 110 )
- The Regulatory Mechanism of Src Familly Kinase in Lipopolysaccharide (LPS) induced HF-kappaB Activation Pathway