Tuberc Respir Dis.
2011 Mar;70(3):206-217. 10.4046/trd.2011.70.3.206.
The Regulation of FOXP3 Expression by the Treatment of TGF-beta and the Modification of DNA Methylation in Lung Cancer Cell Lines
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. sangwonum@skku.edu
- 2Department of Anatomy and Tumor Immunity Medical Research Center, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1442850
- DOI: http://doi.org/10.4046/trd.2011.70.3.206
Abstract
- BACKGROUND
Transcription factor FOXP3 characterizes the thymically derived regulatory T cells. FOXP3 is expressed by cancer cell itself and FOXP3 expression was induced by TGF-beta treatment in pancreatic cancer cell line. However, the expression of FOXP3 expression is not well known in patients with lung cancer. This study was conducted to investigate the expression of FOXP3 in patients with lung cancer and to investigate the regulation of FOXP3 expression by the treatment of TGF-beta and DNA methyltransferase inhibitor in lung cancer cell lines.
METHODS
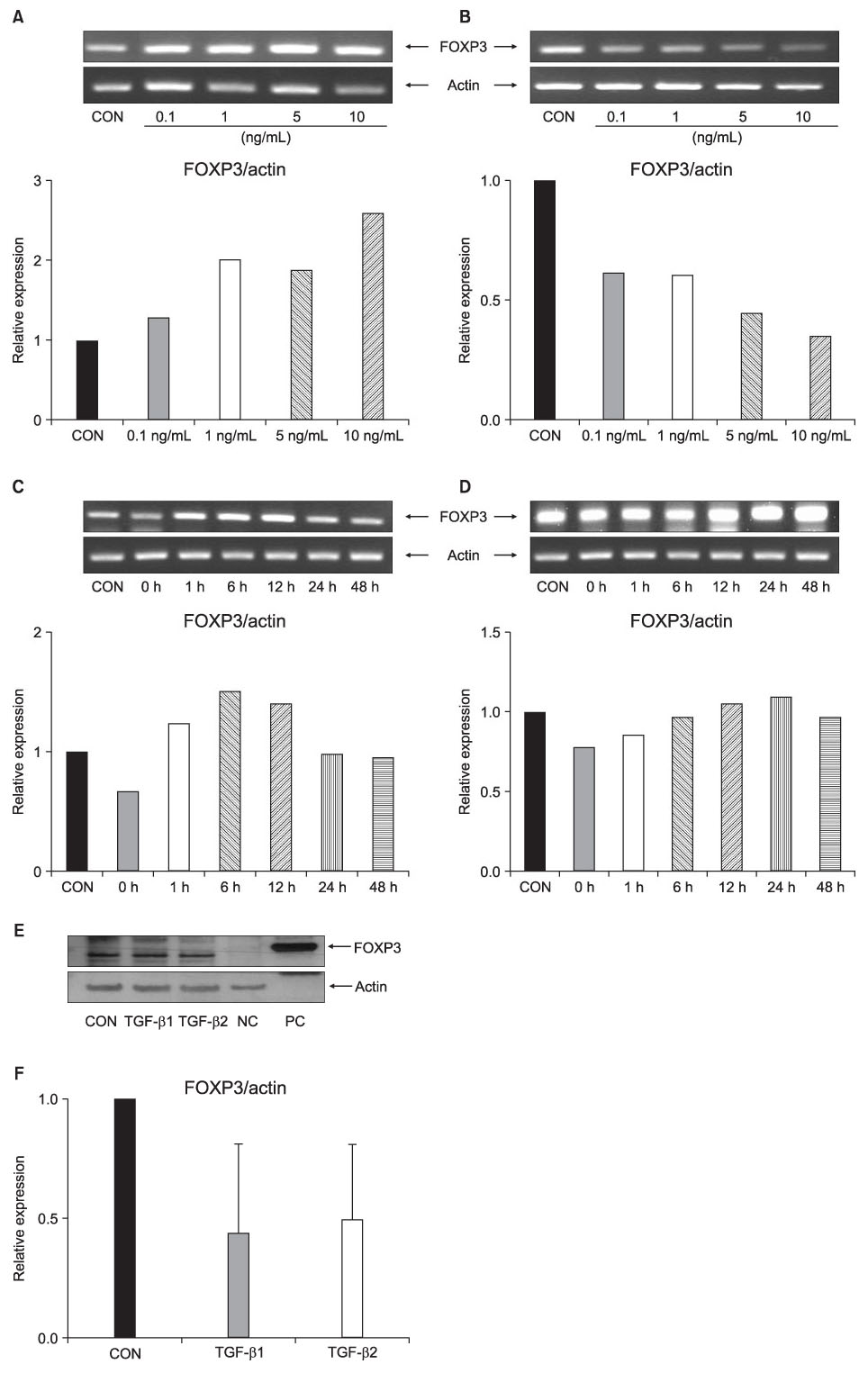
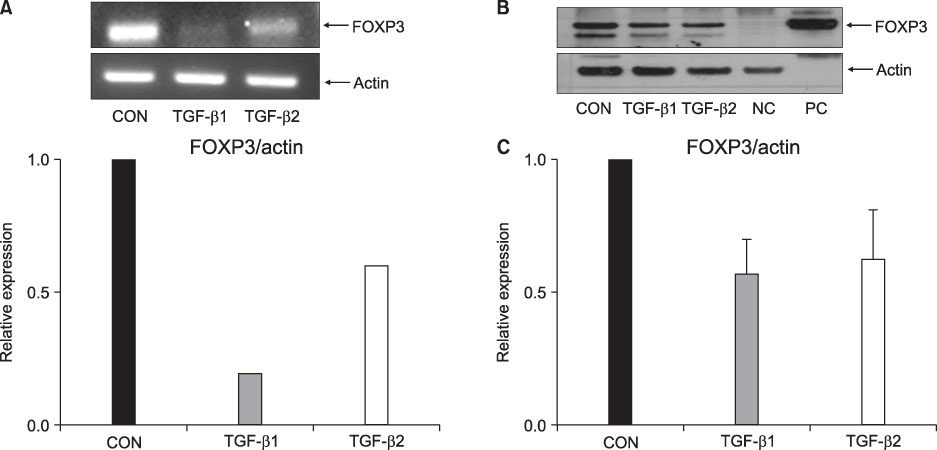
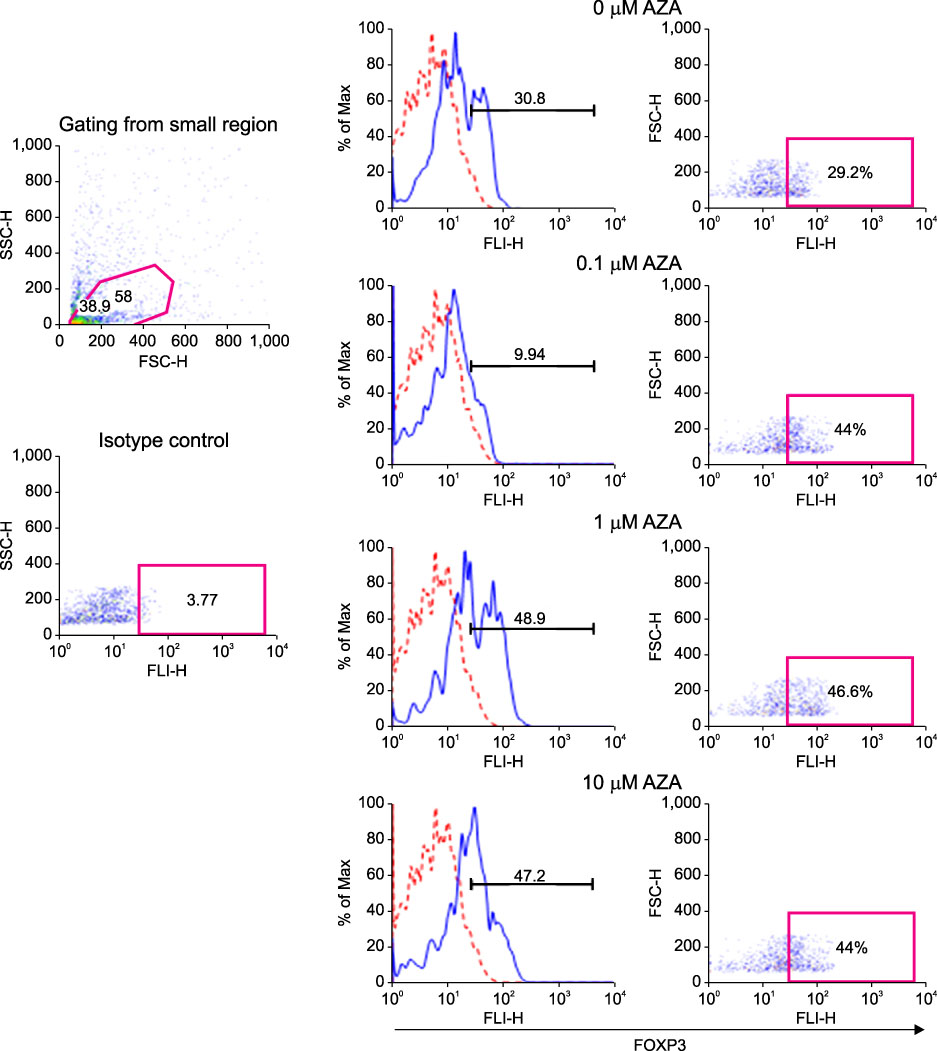
FOXP3 expression in the tissue of patients with resected non-small cell lung cancer (NSCLC) was evaluated by immunohistochemistry. The regulation of FOXP3 expression was investigated by Western blot and RT-PCR after lung cancer cell lines were stimulated with TGF-beta1 and TGF-beta2. The regulation of FOXP3 expression was also investigated by RT-PCR and flow cytometry after lung cancer cell lines were treated with DNA methyltransferase inhibitor (5-AZA-dC).
RESULTS
FOXP3 expression was confirmed in 27% of patients with NSCLC. In NCI-H460 cell line, TGF-beta2 decreased FOXP3 mRNA and protein expressions. In A549 cell line, both TGF-beta1 and TGF-beta2 decreased FOXP3 mRNA and protein expressions. 5-AZA-dC increased FOXP3 mRNA expression in NCI-H460 and A549 cell lines. Moreover, 5-AZA-dC increased intracellular FOXP3 protein expression in A549 cell lines.
CONCLUSION
It was shown that FOXP3 is expressed by cancer cell itself in patients with NSCLC. Treatment of TGF-beta2 and DNA methyltransferase inhibitor seems to be associated with the regulation of FOXP3 expression in lung cancer cell lines.
MeSH Terms
-
Blotting, Western
Carcinoma, Non-Small-Cell Lung
Cell Line
DNA
DNA Methylation
Flow Cytometry
Forkhead Transcription Factors
Humans
Immunohistochemistry
Lung
Lung Neoplasms
Pancreatic Neoplasms
RNA, Messenger
T-Lymphocytes, Regulatory
Transcription Factors
Transforming Growth Factor beta
Transforming Growth Factor beta1
Transforming Growth Factor beta2
DNA
Forkhead Transcription Factors
RNA, Messenger
Transcription Factors
Transforming Growth Factor beta
Transforming Growth Factor beta1
Transforming Growth Factor beta2
Figure
Reference
-
1. Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005. 22:329–341.2. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003. 299:1057–1061.3. Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, et al. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007. 129:1275–1286.4. Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grüssel S, Sipos B, et al. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res. 2007. 67:8344–8350.5. Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Mènard S, et al. FOXP3 expression and overall survival in breast cancer. J Clin Oncol. 2009. 27:1746–1752.6. Zuo T, Liu R, Zhang H, Chang X, Liu Y, Wang L, et al. FOXP3 is a novel transcriptional repressor for the breast cancer oncogene SKP2. J Clin Invest. 2007. 117:3765–3773.7. Wang L, Liu R, Li W, Chen C, Katoh H, Chen GY, et al. Somatic single hits inactivate the X-linked tumor suppressor FOXP3 in the prostate. Cancer Cell. 2009. 16:336–346.8. Ebert LM, Tan BS, Browning J, Svobodova S, Russell SE, Kirkpatrick N, et al. The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res. 2008. 68:3001–3009.9. Karanikas V, Speletas M, Zamanakou M, Kalala F, Loules G, Kerenidi T, et al. Foxp3 expression in human cancer cells. J Transl Med. 2008. 6:19.10. Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007. 204:1543–1551.11. Cui DD, Huang Y, Mao SH, Chen SC, Qiu M, Ji LL, et al. Synergistic antitumor effect of TRAIL and adriamycin on the human breast cancer cell line MCF-7. Braz J Med Biol Res. 2009. 42:854–862.12. Kretzschmar M, Doody J, Timokhina I, Massagué J. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes Dev. 1999. 13:804–816.13. Massagué J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006. 580:2811–2820.14. Park C, Kim WS, Choi Y, Kim H, Park K. Effects of transforming growth factor beta (TGF-beta) receptor on lung carcinogenesis. Lung Cancer. 2002. 38:143–147.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- AKAP12alpha is Associated with Promoter Methylation in Lung Cancer
- The role of promoter methylation in Epstein-Barr virus (EBV) microRNA expression in EBV-infected B cell lines
- Expression of Transforming Growth Factor-alpha and Transforming Growth Factor-beta In Human Primary Lung Cancers
- DNA Methylation of RUNX3 in Papillary Thyroid Cancer
- DNA Methylation in Lung Cancer