J Korean Bone Joint Tumor Soc.
2012 Jun;18(1):1-6. 10.5292/jkbjts.2012.18.1.1.
Outcomes of Treatment for Squamous Cell Carcinoma Originating as a Marjolin's Ulcer
- Affiliations
-
- 1Department of Orthopaedic Surgery, Medical School, Chonbuk National University, Jeonju, Korea.
- 2Department of Orthopaedic Surgery, Presbyterian Medical Center, Jeonju, Korea. jrkeem@jbnu.ac.kr
- KMID: 1438397
- DOI: http://doi.org/10.5292/jkbjts.2012.18.1.1
Abstract
- PURPOSE
The purpose of this study was to analyze the results of treatment and prognosis of Marjolin's ulcer compared with primary squamous cell carcinoma.
MATERIALS AND METHODS
Fourteen patients treated for Marjolin's ulcer were analyzed. Twenty patients with primary squamous cell carcinoma treated during the same time period was the control group. Mean age was 61.2 years. There were 24 males and 10 females. The locations, TNM stages, histological grades, recurrence, metastasis, and survival rate were analyzed and compared between two groups.
RESULTS
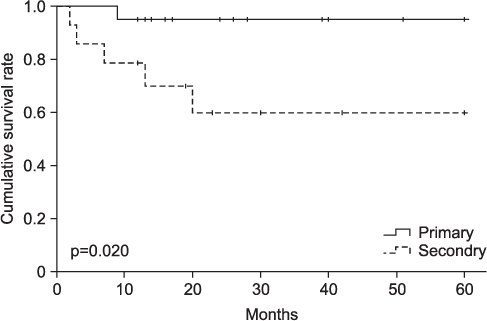
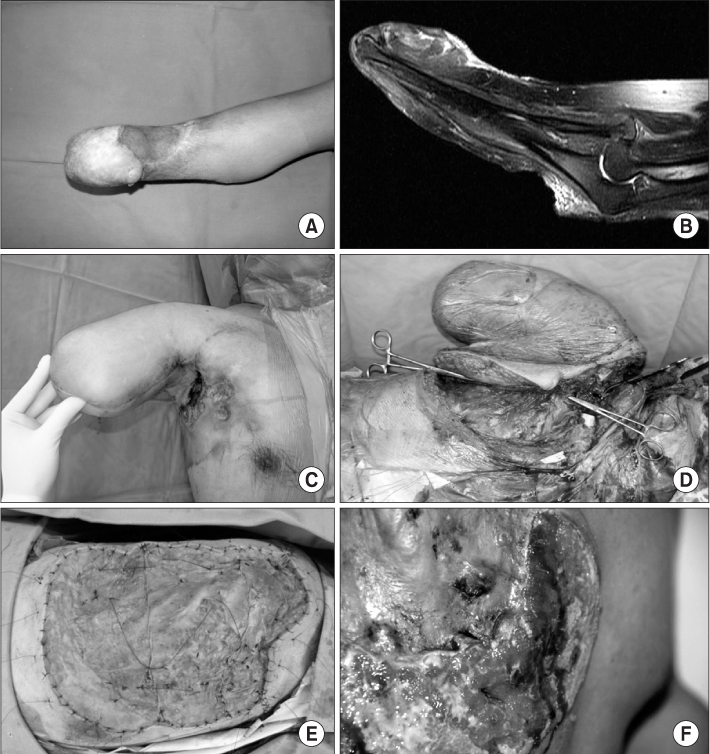
The mean follow-up period was 54.8 months (range, 12-168 months). Local recurrences were found in 6 cases, 5 ones in Marjolin's ulcer patients, and one case in primary squamous cell carcinoma patients. The mean time interval between the initial presentation and occurrence of local recurrences was 9 months (range, 2-20 months). There were 6 metastases. 2 (14.3%) metastases were found in Marjolin's ulcer patients, and 4 (20.0%) metastases in primary squamous cell carcinoma patients. Total events (metastasis or local recurrence) were found in 10 pateients, 6 of them in Marjolin's ulcer group, and the remaining four in primary group. 5-year disease-free survival rate was 64.3% in Marjolin's ulcer group and 95.0% in primary squamous cell carcinoma group.
CONCLUSION
Squamous cell carcinomas originating as Marjolin's ulcers revealed higher recurrence rate and lower survival rate despite of aggressive treatment. Therefore, new treatment modalities should be developed for improving outcomes.
MeSH Terms
Figure
Reference
-
1. Hejna WF. Squamous-cell carcinoma developing in the chronic draining sinuses of osteomyelitis. Cancer. 1965. 18:128–132.2. Kaplan RP. Cancer complicating chronic ulcerative and scarifying mucocutaneous disorders. Adv Dermatol. 1987. 2:19–46.3. Chuang TY, Popescu NA, Su WP, Chute CG. Squamous cell carcinoma. A population-based incidence study in Rochester, Minn. Arch Dermatol. 1990. 126:185–188.4. Edwards EK Jr, Edwards EK Sr. Superficial x-ray treatment of squamous cell carcinoma of the lower extremity. Cutis. 1991. 48:240.5. Lifeso RM, Rooney RJ, el-Shaker M. Post-traumatic squamous-cell carcinoma. J Bone Joint Surg Am. 1990. 72:12–18.6. Kwa RE, Campana K, Moy RL. Biology of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1992. 26:1–26.7. Crawley WA, Dellon AL, Ryan JJ. Does host response determine the prognosis in scar carcinoma? Plast Reconstr Surg. 1978. 62:407–414.8. Patel NM, Weiner SD, Senior M. Squamous cell carcinoma arising from chronic osteomyelitis of the patella. Orthopedics. 2002. 25:334–336.9. Edwards MJ, Hirsch RM, Broadwater JR, Netscher DT, Ames FC. Squamous cell carcinoma arising in previously burned or irradiated skin. Arch Surg. 1989. 124:115–117.10. Jeon DG, Lee JS, Kim SJ, et al. Role of surgery in squamous cell carcinoma. J Korean Bone Joint Tumor Soc. 1998. 4:30–36.11. Sirsat MV, Shrikhande SS. Histochemical studies on squamous cell carcinoma of the skin arising in burn scars with special reference to histogenesis. Indian J Cancer. 1966. 3:157–169.12. Sedlin ED, Fleming JL. Epidermoid carcinoma arising in chronic osteomyelitis foci. J Bone Joint Surg Am. 1963. 45:827–838.13. Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992. 26:976–990.14. McGrory JE, Pritchard DJ, Unni KK, Ilstrup D, Rowland CM. Malignant lesions arising in chronic osteomyelitis. Clin Orthop Relat Res. 1999. (362):181–189.15. Eroğlu A, Camlibel S. Risk factors for locoregional recurrence of scar carcinoma. Br J Surg. 1997. 84:1744–1746.16. Wheeler RH, Baker SR. Lokich JJ, editor. Head and neck cancer. Cancer chemotherapy by infusion. 1987. Chicago: Percept Press;110.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Marjolin's Ulcer Presenting with In-Transit Metastases: A Case Report and Literature Review
- Extensive Squamous Cell Carcinoma Originating from a Burn Scar: Marjolin's Ulcer with an Unusual Presentation
- A clinical analysis of the marjolin's ulcer
- Squamous Cell Carcinoma Occurring at the Site of an Arteriovenous Fistula Ulcer: A Case Report
- Squamous Cell Carcinoma Showing Rapid Metastasis after Leg Amputation due to Chronic Osteomyelitis