Hanyang Med Rev.
2012 Aug;32(3):145-153. 10.7599/hmr.2012.32.3.145.
Treatment Strategy for Parkinsonian Diseases Through Mesenchymal Stem Cells
- Affiliations
-
- 1Department of Neurology and Brain Research Institute, Yonsei University College of Medicine, Severance Biomedical Science Institute, Yonsei University, Seoul, Korea. phisland@chol.net
- KMID: 1436766
- DOI: http://doi.org/10.7599/hmr.2012.32.3.145
Abstract
- Parkinsonian diseases including Parkinson's disease (PD) and multiple system atrophy (MSA) are neurodegenerative diseases representative of alpha-synucleinopathies characterized pathologically by alpha-synuclein-abundant Lewy bodies and glial cytoplasmic inclusions, respectively. Cell therapy using mesenchymal stem cells (MSCs) is attractive clinically because these cells are free from ethical and immunological problems. MSCs are present in adult bone marrow and represent <0.01% of all nucleated bone marrow cells. MSCs are multipotent, and differentiation under appropriate conditions into chondrocytes, skeletal myocytes, and neurons has been demonstrated thus far. According to recent studies, the neuroprotective effect of MSCs is mediated by the production of various trophic factors that contribute to functional recovery, neuronal cell survival, and endogenous regeneration of neural tissues. Additionally, MSCs appear to have immunoregulatory properties that can ameliorate the progression of disease. However, the therapeutic use of MSCs as neuroprotectives in PD and MSA has seldom been studied. Here we comprehensively review recent advances in clinical strategies using MSCs in PD and MSA, especially focusing on their neuroprotective properties in preventing or delaying disease progression and therapeutic potential for providing functional recovery.
MeSH Terms
-
Adult
Bone Marrow
Bone Marrow Cells
Cell Survival
Chondrocytes
Disease Progression
Humans
Inclusion Bodies
Lewy Bodies
Mesenchymal Stromal Cells
Multiple System Atrophy
Muscle Fibers, Skeletal
Neurodegenerative Diseases
Neurons
Neuroprotective Agents
Parkinson Disease
Parkinsonian Disorders
Regeneration
Tissue Therapy
Neuroprotective Agents
Figure
Reference
-
1. Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005. 28:57–87.
Article2. von Bohlen und Halbach O, Schober A, Krieglstein K. Genes, proteins, and neurotoxins involved in Parkinson's disease. Prog Neurobiol. 2004. 73:151–177.
Article3. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article4. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.
Article5. Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood). 2001. 226:507–520.
Article6. Bonuccelli U, Del Dotto P. New pharmacologic horizons in the treatment of Parkinson disease. Neurology. 2006. 67:S30–S38.
Article7. Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004. 36:568–584.
Article8. Krampera M, Pasini A, Pizzolo G, Cosmi L, Romagnani S, Annunziato F. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin Pharmacol. 2006. 6:435–441.
Article9. Karussis D, Kassis I, Kurkalli BG, Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci. 2008. 265:131–135.
Article10. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007. 110:3499–3506.
Article11. Blondheim NR, Levy YS, Ben-Zur T, Burshtein A, Cherlow T, Kan I, et al. Human mesenchymal stem cells express neural genes, suggesting a neural predisposition. Stem Cells Dev. 2006. 15:141–164.
Article12. Barzilay R, Kan I, Ben-Zur T, Bulvik S, Melamed E, Offen D. Induction of human mesenchymal stem cells into dopamine-producing cells with different differentiation protocols. Stem Cells Dev. 2008. 17:547–554.
Article13. Offen D, Barhum Y, Levy YS, Burshtein A, Panet H, Cherlow T, et al. Intrastriatal transplantation of mouse bone marrow-derived stem cells improves motor behavior in a mouse model of Parkinson's disease. J Neural Transm Suppl. 2007. 133–143.
Article14. Ye M, Wang XJ, Zhang YH, Lu GQ, Liang L, Xu JY, et al. Therapeutic effects of differentiated bone marrow stromal cell transplantation on rat models of Parkinson's disease. Parkinsonism Relat Disord. 2007. 13:44–49.
Article15. Park HJ, Lee PH, Bang OY, Lee G, Ahn YH. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson's disease. J Neurochem. 2008. 107:141–151.
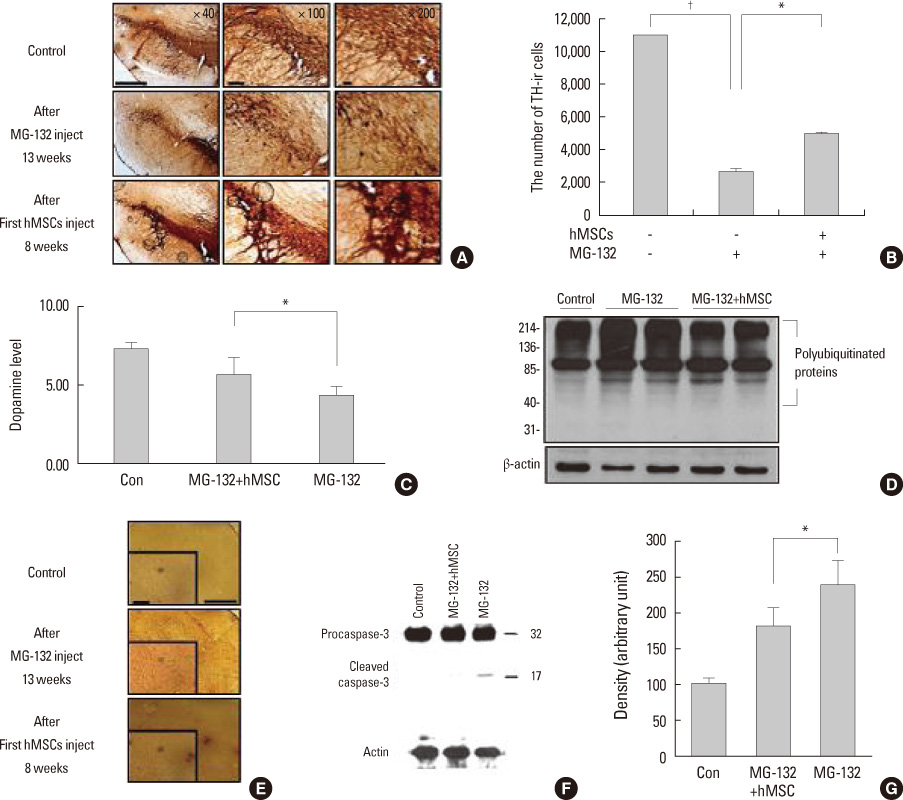
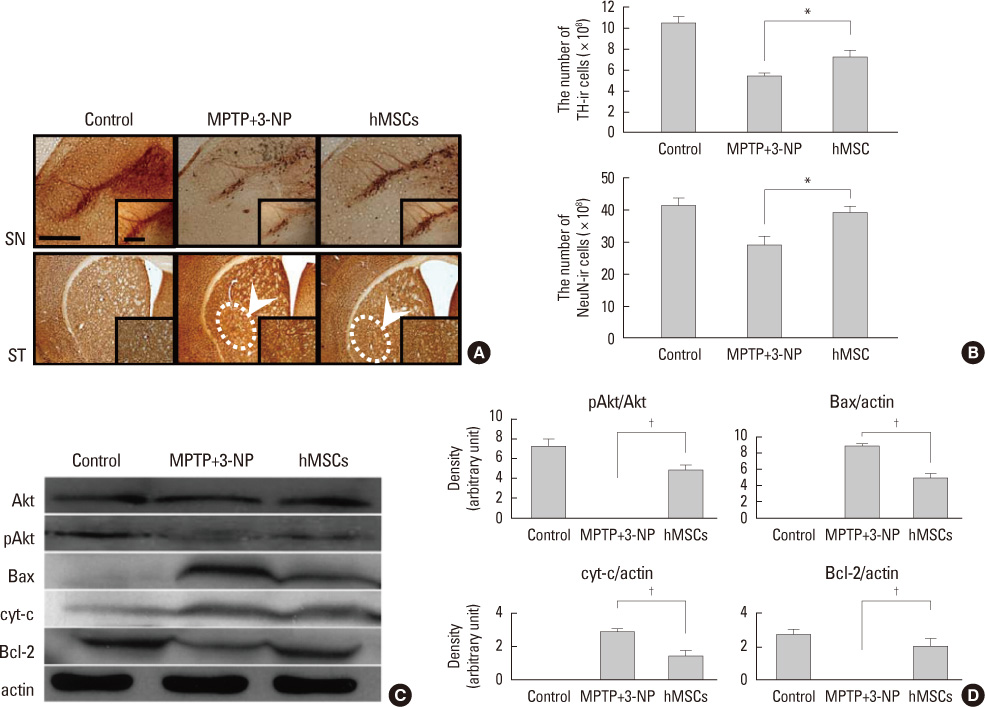
Article16. Park HJ, Bang G, Lee BR, Kim HO, Lee PH. Neuroprotective effect of human mesenchymal stem cells in an animal model of double toxin-induced multiple system atrophy parkinsonism. Cell Transplant. 2011. 20:827–835.
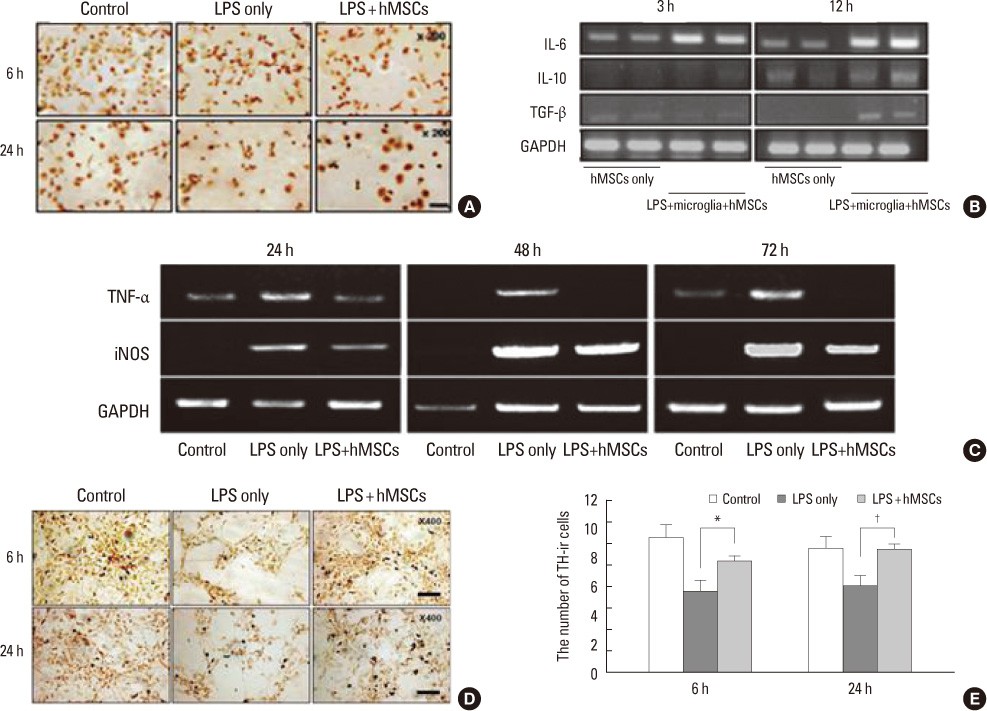
Article17. Kim YJ, Park HJ, Lee G, Bang OY, Ahn YH, Joe E, et al. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009. 57:13–23.
Article18. Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998. 4:1313–1317.
Article19. Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993. 11:173–189.
Article20. Doetsch F, Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Curr Opin Neurobiol. 2005. 15:121–128.21. Borta A, Hoglinger GU. Dopamine and adult neurogenesis. J Neurochem. 2007. 100:587–595.22. Crews L, Mizuno H, Desplats P, Rockenstein E, Adame A, Patrick C, et al. Alpha-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J Neurosci. 2008. 28:4250–4260.
Article23. Winner B, Rockenstein E, Lie DC, Aigner R, Mante M, Bogdahn U, et al. Mutant alpha-synuclein exacerbates age-related decrease of neurogenesis. Neurobiol Aging. 2008. 29:913–925.
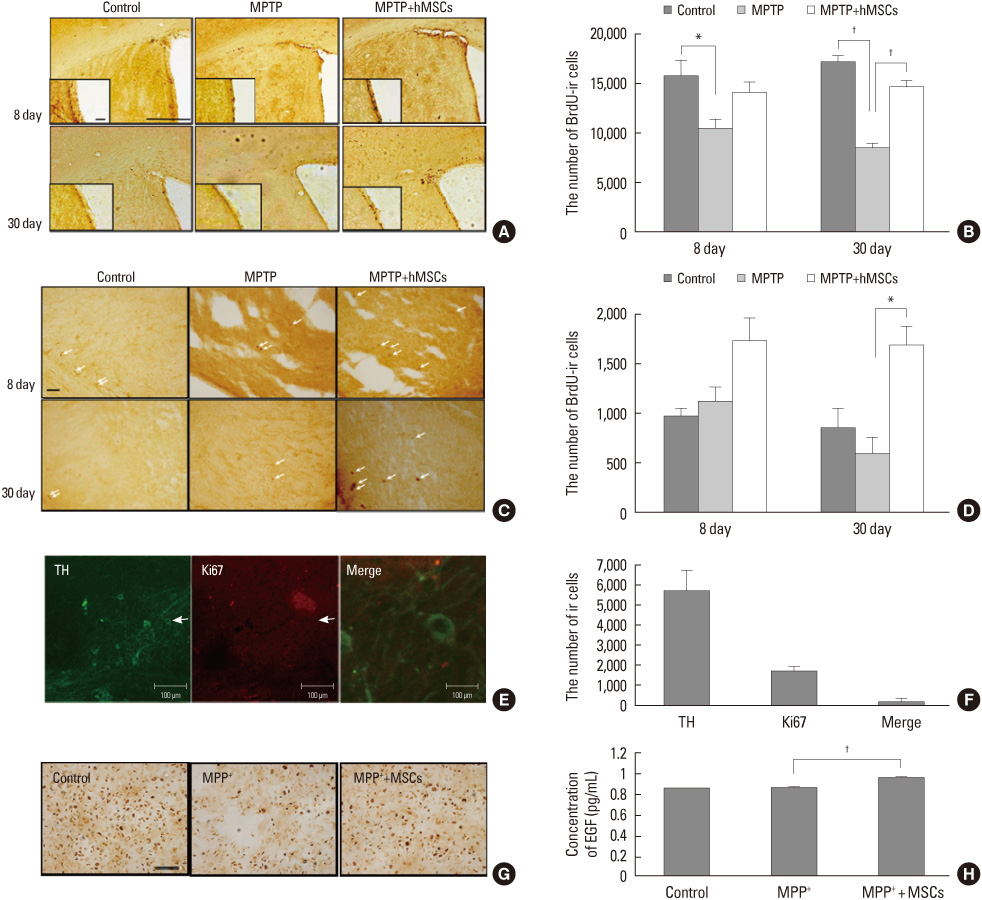
Article24. Park HJ, Shin JY, Lee BR, Kim HO, Lee PH. Mesenchymal stem cells augment neurogenesis in the subventricular zone and enhance differentiation of neural precursor cells into dopaminergic neurons in the substantia nigra of a Parkinsonian model. Cell Transplant. Forthcoming 2012.
Article25. Lee PH, Lee JE, Kim HS, Song SK, Lee HS, Nam HS, et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012. 72:32–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Trends and Prospect of Cell Therapy using Hematopoietic Stem Cells
- Strategy for Maximizing Therapeutic Efficacy of Adult Stem Cells
- Adult Stem Cells: Beyond Regenerative Tool, More as a Bio-Marker in Obesity and Diabetes
- Stem Cells and Niemann Pick Disease
- Recent Trends and Strategies in Stem Cell Therapy for Alzheimer's Disease