Lab Anim Res.
2012 Sep;28(3):171-179. 10.5625/lar.2012.28.3.171.
Prophylactic effects of swimming exercise on autophagy-induced muscle atrophy in diabetic rats
- Affiliations
-
- 1Department of Rehabilitation Science in Interdisciplinary PhD Program, Inje University, Gimhae, Korea. yonghong@inje.ac.kr
- 2National Primate Research Center (NPRC), Korea Research Institute of Bioscience and Biotechnology (KRIBB), Ochang, Korea.
- 3Institute of Animal Medicine, College of Veterinary Medicine, Gyeongsang National University, Jinju, Korea.
- 4Cardiovascular & Metabolic Disease Center, College of Biomedical Science & Engineering, Inje University, Gimhae, Korea.
- KMID: 1436722
- DOI: http://doi.org/10.5625/lar.2012.28.3.171
Abstract
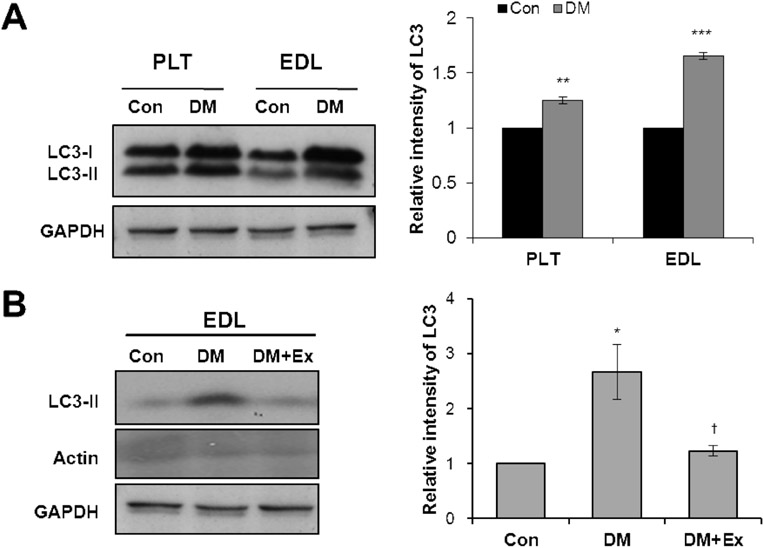
- Diabetes decreases skeletal muscle mass and induces atrophy. However, the mechanisms by which hyperglycemia and insulin deficiency modify muscle mass are not well defined. In this study, we evaluated the effects of swimming exercise on muscle mass and intracellular protein degradation in diabetic rats, and proposed that autophagy inhibition induced by swimming exercise serves as a hypercatabolic mechanism in the skeletal muscles of diabetic rats, supporting a notion that swimming exercise could efficiently reverse the reduced skeletal muscle mass caused by diabetes. Adult male Sprague-Dawley rats were injected intraperitoneally with streptozotocin (60 mg/kg body weight) to induce diabetes and then submitted to 1 hr per day of forced swimming exercise, 5 days per week for 4 weeks. We conducted an intraperitoneal glucose tolerance test on the animals and measured body weight, skeletal muscle mass, and protein degradation and examined the level of autophagy in the isolated extensor digitorum longus, plantaris, and soleus muscles. Body weight and muscle tissue mass were higher in the exercising diabetic rats than in control diabetic rats that remained sedentary. Compared to control rats, exercising diabetic rats had lower blood glucose levels, increased intracellular contractile protein expression, and decreased autophagic protein expression. We conclude that swimming exercise improves muscle mass in diabetes-induced skeletal muscle atrophy, suggesting the activation of autophagy in diabetes contributes to muscle atrophy through hypercatabolic metabolism and that aerobic exercise, by suppressing autophagy, may modify or reverse skeletal muscle wasting in diabetic patients.
MeSH Terms
Figure
Reference
-
1. Sun Z, Liu L, Liu N, Liu Y. Muscular response and adaptation to diabetes mellitus. Front Biosci. 2008. 13:4765–4794.2. Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999. 129:1S Suppl. 227S–237S.3. Emery PW. Cachexia in experimental models. Nutrition. 1999. 15(7-8):600–603.4. Costelli P, Baccino FM. Cancer cachexia: from experimental models to patient management. Curr Opin Clin Nutr Metab Care. 2000. 3(3):177–181.5. Attaix D, Combaret L, Tilignac T, Taillandier D. Adaptation of the ubiquitin-proteasome proteolytic pathway in cancer cachexia. Mol Biol Rep. 1999. 26(1-2):77–82.6. Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004. 6(4):463–477.7. Meijer AJ, Codogno P. Autophagy: a sweet process in diabetes. Cell Metab. 2008. 8(4):275–276.8. Bird JW. Skeletal muscle lysosomes. Front Biol. 1975. 43(4):75–109.9. Gerard KW, Hipkiss AR, Schneider DL. Degradation of intracellular protein in muscle. Lysosomal response to modified proteins and chloroquine. J Biol Chem. 1988. 263(35):18886–18890.10. Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000. 290(5497):1717–1721.11. Cuervo AM. Autophagy: many paths to the same end. Mol Cell Biochem. 2004. 263(1-2):55–72.12. Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004. 36(6):585–595.13. Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004. 119(6):753–766.14. Xue L, Fletcher GC, Tolkovsky AM. Mitochondria are selectively eliminated from eukaryotic cells after blockade of caspases during apoptosis. Curr Biol. 2001. 11(5):361–365.15. Mizushima N, Noda T, Ohsumi Y. Apg16p is required for the function of the Apg12p-Apg5p conjugate in the yeast autophagy pathway. EMBO J. 1999. 18(14):3888–3896.16. Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001. 152(4):657–668.17. Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T, Ohsumi M, Takao T, Noda T, Ohsumi Y. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol. 2000. 151(2):263–276.18. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000. 19(21):5720–5728.19. Broderick TL, Poirier P, Gillis M. Exercise training restores abnormal myocardial glucose utilization and cardiac function in diabetes. Diabetes Metab Res Rev. 2005. 21(1):44–50.20. Farrell PA, Fedele MJ, Hernandez J, Fluckey JD, Miller JL 3rd, Lang CH, Vary TC, Kimball SR, Jefferson LS. Hypertrophy of skeletal muscle in diabetic rats in response to chronic resistance exercise. J Appl Physiol. 1999. 87(3):1075–1082.21. Wegner JA, Lund DD, Overton JM, Edwards JG, Oda RP, Tipton CM. Select cardiovascular and metabolic responses of diabetic rats to moderate exercise training. Med Sci Sports Exerc. 1987. 19(5):497–503.22. Gomez R, Asnis N, Tannhauser SL, Barros HM. GABA agonists differentially modify blood glucose levels of diabetic rats. Jpn J Pharmacol. 1999. 80(4):327–331.23. Ward DT, Yau SK, Mee AP, Mawer EB, Miller CA, Garland HO, Riccardi D. Functional, molecular, and biochemical characterization of streptozotocin-induced diabetes. J Am Soc Nephrol. 2001. 12(4):779–790.24. Garland HO, Hamilton K, Freeman S, Burns C, Cusack M, Balment RJ. Renal function in chronically catheterized conscious diabetic rats using constant and servo-controlled infusion. Clin Exp Pharmacol Physiol. 1999. 26(10):803–808.25. Harri M, Kuusela P. Is swimming exercise or cold exposure for rats? Acta Physiol Scand. 1986. 126(2):189–197.26. Gobatto CA, de Mello MA, Sibuya CY, de Azevedo JR, dos Santos LA, Kokubun E. Maximal lactate steady state in rats submitted to swimming exercise. Comp Biochem Physiol A Mol Integr Physiol. 2001. 130(1):21–27.27. Király MA, Bates HE, Yue JT, Goche-Montes D, Fediuc S, Park E, Matthews SG, Vranic M, Riddell MC. Attenuation of type 2 diabetes mellitus in the male Zucker diabetic fatty rat: the effects of stress and non-volitional exercise. Metabolism. 2007. 56(6):732–744.28. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–254.29. Trujillo-Santos AJ. Diabetic muscle infarction: an underdiagnosed complication of long-standing diabetes. Diabetes Care. 2003. 26(1):211–215.30. Röckl KS, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life. 2008. 60(3):145–153.31. Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967. 242(9):2278–2282.32. Gollnick PD, Armstrong RB, Saubert CW 4th, Piehl K, Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol. 1972. 33(3):312–319.33. Goodyear LJ, Giorgino F, Balon TW, Condorelli G, Smith RJ. Effects of contractile activity on tyrosine phosphoproteins and PI 3-kinase activity in rat skeletal muscle. Am J Physiol. 1995. 268(5 Pt 1):E987–E995.34. Treadway JL, James DE, Burcel E, Ruderman NB. Effect of exercise on insulin receptor binding and kinase activity in skeletal muscle. Am J Physiol. 1989. 256(1 Pt 1):E138–E144.35. Sandström ME, Zhang SJ, Bruton J, Silva JP, Reid MB, Westerblad H, Katz A. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J Physiol. 2006. 575(Pt 1):251–262.36. Balon TW, Nadler JL. Evidence that nitric oxide increases glucose transport in skeletal muscle. J Appl Physiol. 1997. 82(1):359–363.37. Mu J, Brozinick JT Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001. 7(5):1085–1094.38. Ponticos M, Lu QL, Morgan JE, Hardie DG, Partridge TA, Carling D. Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. EMBO J. 1998. 17(6):1688–1699.39. Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5' AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998. 47(8):1369–1373.40. Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005. 6(6):439–448.41. Hein S, Arnon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, Bauer EP, Klövekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003. 107(7):984–991.42. Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005. 102(39):13807–13812.43. Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001. 294(5544):1102–1105.44. Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007. 100(6):914–922.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Exercise on Neuropathy in Streptozotocin-Induced Diabetic Rats
- Aerobic Exercise Ameliorates Muscle Atrophy Induced by Methylglyoxal via Increasing Gastrocnemius and Extensor Digitorum Longus Muscle Sensitivity
- Protective Effect of Exercise on Peripheral Nerve Injury Induced by Ischemia/Reperfusion in Rats
- Modulation of transglutaminase expression in rat skeletal muscle by induction of atrophy and endurance training
- Effect of Exercise on Antioxidant Enzyme Activities of Skeletal Muscle and Liver in STZ-diabetic Rats