Lab Anim Res.
2012 Jun;28(2):71-76. 10.5625/lar.2012.28.2.71.
Functions and physiological roles of two types of estrogen receptors, ERalpha and ERbeta, identified by estrogen receptor knockout mouse
- Affiliations
-
- 1Laboratory of Veterinary Biochemistry and Immunology, College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea. kchoi@cbu.ac.kr
- 2Department of Obstetrics and Gynecology, College of Medicine, Soonchunhyang University, Bucheon, Korea.
- KMID: 1436696
- DOI: http://doi.org/10.5625/lar.2012.28.2.71
Abstract
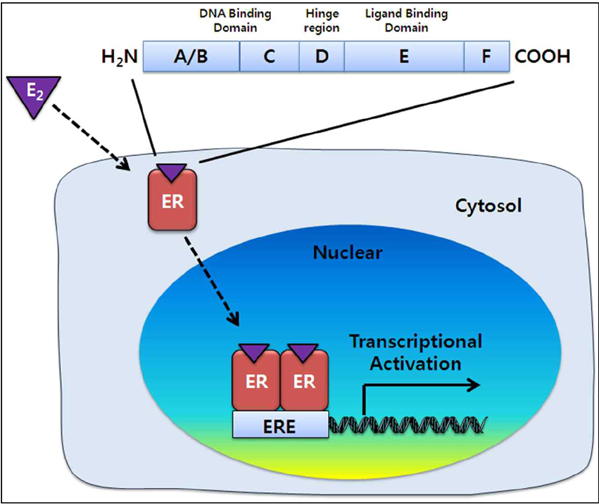
- Estrogens, a class of steroid hormones, regulate the growth, development, and physiology of the human reproductive system. Estrogens also involve in the neuroendocrine, skeletal, adipogenesis, and cardiovascular systems. Estrogen signaling pathways are selectively stimulated or inhibited depending on a balance between the activities of estrogen receptor (ER) alpha or ERbeta in target organs. ERs belong to the steroid hormone superfamily of nuclear receptors, which act as transcription factors after binding to estrogen. The gene expression regulation by ERs is to modulate biological activities, such as reproductive organ development, bone modeling, cardiovascular system functioning, metabolism, and behavior in both females and males. Understanding of the general physiological roles of ERs has been gained when estrogen levels were ablated by ovariectomy and then replenished by treatment with exogenous estrogen. This technique is not sufficient to fully determine the exact function of estrogen signaling in general processes in living tissues. However, a transgenic mouse model has been useful to study gene-specific functions. ERalpha and ERbeta have different biological functions, and knockout and transgenic animal models have distinct phenotypes. Analysis of ERalpha and ERbeta function using knockout mouse models has identified the roles of estrogen signaling in general physiologic processes. Although transgenic mouse models do not always produce consistent results, they are the useful for studying the functions of these genes under specific pathological conditions.
Keyword
MeSH Terms
-
Adipogenesis
Animals
Animals, Genetically Modified
Cardiovascular System
Estrogen Receptor alpha
Estrogen Receptor beta
Estrogens
Female
Gene Expression Regulation
Humans
Male
Mice
Mice, Knockout
Mice, Transgenic
Ovariectomy
Phenotype
Receptors, Cytoplasmic and Nuclear
Receptors, Estrogen
Transcription Factors
Estrogen Receptor alpha
Estrogen Receptor beta
Estrogens
Receptors, Cytoplasmic and Nuclear
Receptors, Estrogen
Transcription Factors
Figure
Reference
-
1. Osz J, Brelivet Y, Peluso-Iltis C, Cura V, Eiler S, Ruff M, Bourguet W, Rochel N, Moras D. Structural basis for a molecular allosteric control mechanism of cofactor binding to nuclear receptors. Proc Natl Acad Sci U S A. 2012. 109(10):E588–E594.2. Choi KC, Jeung EB. The biomarker and endocrine disruptors in mammals. J Reprod Dev. 2003. 49(5):337–345.3. Weigel NL, Moore NL. Cyclins, cyclin dependent kinases, and regulation of steroid receptor action. Mol Cell Endocrinol. 2007. 265-266:157–161.4. Lee HR, Hwang KA, Park MA, Yi BR, Jeung EB, Choi KC. Treatment with bisphenol A and methoxychlor results in the growth of human breast cancer cells and alteration of the expression of cell cycle-related genes, cyclin D1 and p21, via an estrogen receptor-dependent signaling pathway. Int J Mol Med. 2012. 29(5):883–890.5. Swedenborg E, Power KA, Cai W, Pongratz I, Ruegg J. Regulation of estrogen receptor beta activity and implications in health and disease. Cell Mol Life Sci. 2009. 66(24):3873–3894.6. Hughes ZA, Liu F, Marquis K, Muniz L, Pangalos MN, Ring RH, Whiteside GT, Brandon NJ. Estrogen receptor neurobiology and its potential for translation into broad spectrum therapeutics for CNS disorders. Curr Mol Pharmacol. 2009. 2(3):215–236.7. Xiao J, Wang NL, Sun B, Cai GP. Estrogen receptor mediates the effects of pseudoprotodiocsin on adipogenesis in 3T3-L1 cells. Am J Physiol Cell Physiol. 2010. 299(1):C128–C138.8. Welboren WJ, Sweep FC, Span PN, Stunnenberg HG. Genomic actions of estrogen receptor alpha: what are the targets and how are they regulated? Endocr Relat Cancer. 2009. 16(4):1073–1089.9. Kong EH, Pike AC, Hubbard RE. Structure and mechanism of the oestrogen receptor. Biochem Soc Trans. 2003. 31(Pt 1):56–59.10. Skafar DF, Zhao C. The multifunctional estrogen receptor-alpha F domain. Endocrine. 2008. 33(1):1–8.11. Geserick C, Meyer HA, Haendler B. The role of DNA response elements as allosteric modulators of steroid receptor function. Mol Cell Endocrinol. 2005. 236(1-2):1–7.12. Edwards DP. The role of coactivators and corepressors in the biology and mechanism of action of steroid hormone receptors. J Mammary Gland Biol Neoplasia. 2000. 5(3):307–324.13. Sanchez ER. Chaperoning steroidal physiology: Lessons from mouse genetic models of Hsp90 and its cochaperones. Biochim Biophys Acta. 2012. 1823(3):722–729.14. Beliakoff J, Whitesell L. Hsp90: an emerging target for breast cancer therapy. Anticancer Drugs. 2004. 15(7):651–662.15. McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol. 2008. 290(1-2):24–30.16. Park MA, Hwang KA, Choi KC. Diverse animal models to examine potential role(s) and mechanism of endocrine disrupting chemicals on the tumor progression and prevention: Do they have tumorigenic or anti-tumorigenic property? Lab Anim Res. 2011. 27(4):265–273.17. Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen signaling via estrogen receptor {beta}. J Biol Chem. 2010. 285(51):39575–39579.18. Zhao C, Dahlman-Wright K, Gustafsson JA. Estrogen receptor beta: an overview and update. Nucl Recept Signal. 2008. 6:e003.19. Lane PH. Estrogen receptors in the kidney: lessons from genetically altered mice. Gend Med. 2008. 5:Suppl A. S11–S18.20. Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: from form to function. Brain Res Rev. 2008. 57(2):309–320.21. Hwang KA, Park SH, Yi BR, Choi KC. Gene alterations of ovarian cancer cells expressing estrogen receptors by estrogen and bisphenol a using microarray analysis. Lab Anim Res. 2011. 27(2):99–107.22. Chen M, Wolfe A, Wang X, Chang C, Yeh S, Radovick S. Generation and characterization of a complete null estrogen receptor alpha mouse using Cre/LoxP technology. Mol Cell Biochem. 2009. 321(1-2):145–153.23. Jayachandran M, Preston CC, Hunter LW, Jahangir A, Owen WG, Korach KS, Miller VM. Loss of estrogen receptor beta decreases mitochondrial energetic potential and increases thrombogenicity of platelets in aged female mice. Age (Dordr). 2010. 32(1):109–121.24. Singh SP, Wolfe A, Ng Y, DiVall SA, Buggs C, Levine JE, Wondisford FE, Radovick S. Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor alpha (ESR1). Biol Reprod. 2009. 81(3):488–496.25. Santollo J, Eckel LA. Effect of a putative ERalpha antagonist, MPP, on food intake in cycling and ovariectomized rats. Physiol Behav. 2009. 97(2):193–198.26. Shao R. Understanding the mechanisms of human tubal ectopic pregnancies: new evidence from knockout mouse models. Hum Reprod. 2010. 25(3):584–587.27. Bockamp E, Sprengel R, Eshkind L, Lehmann T, Braun JM, Emmrich F, Hengstler JG. Conditional transgenic mouse models: from the basics to genome-wide sets of knockouts and current studies of tissue regeneration. Regen Med. 2008. 3(2):217–235.28. Sun J, Langer WJ, Devish K, Lane PH. Compensatory kidney growth in estrogen receptor-alpha null mice. Am J Physiol Renal Physiol. 2006. 290(2):F319–F323.29. Chen M, Hsu I, Wolfe A, Radovick S, Huang K, Yu S, Chang C, Messing EM, Yeh S. Defects of prostate development and reproductive system in the estrogen receptor-alpha null male mice. Endocrinology. 2009. 150(1):251–259.30. Choleris E, Clipperton AE, Phan A, Kavaliers M. Estrogen receptor beta agonists in neurobehavioral investigations. Curr Opin Investig Drugs. 2008. 9(7):760–773.31. Wintermantel TM, Elzer J, Herbison AE, Fritzemeier KH, Schutz G. Genetic dissection of estrogen receptor signaling in vivo. Ernst Schering Found Symp Proc. 2006. (1):25–44.32. Lee GS, Kim HJ, Jung YW, Choi KC, Jeung EB. Estrogen receptor alpha pathway is involved in the regulation of Calbindin-D9k in the uterus of immature rats. Toxicol Sci. 2005. 84(2):270–277.33. An BS, Choi KC, Hong EJ, Jung YW, Manabe N, Jeung EB. Differential transcriptional and translational regulations of calbindin-D9k by steroid hormones and their receptors in the uterus of immature mice. J Reprod Dev. 2004. 50(4):445–453.34. Lee S, Kang DW, Hudgins-Spivey S, Krust A, Lee EY, Koo Y, Cheon Y, Gye MC, Chambon P, Ko C. Theca-specific estrogen receptor-alpha knockout mice lose fertility prematurely. Endocrinology. 2009. 150(8):3855–3862.35. Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci U S A. 2007. 104(19):8173–8177.36. Drummond AE, Fuller PJ. The importance of ERbeta signalling in the ovary. J Endocrinol. 2010. 205(1):15–23.37. Silberstein GB, Van Horn K, Hrabeta-Robinson E, Compton J. Estrogen-triggered delays in mammary gland gene expression during the estrous cycle: evidence for a novel timing system. J Endocrinol. 2006. 190(2):225–239.38. Hewitt SC, Korach KS. Oestrogen receptor knockout mice: roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction. 2003. 125(2):143–149.39. McPherson SJ, Ellem SJ, Risbridger GP. Estrogen-regulated development and differentiation of the prostate. Differentiation. 2008. 76(6):660–670.40. Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J Neurosci. 2009. 29(14):4461–4470.41. Lee KH, Park JH, Bunick D, Lubahn DB, Bahr JM. Morphological comparison of the testis and efferent ductules between wild-type and estrogen receptor alpha knockout mice during postnatal development. J Anat. 2009. 214(6):916–925.42. Imai Y, Kondoh S, Kouzmenko A, Kato S. Regulation of bone metabolism by nuclear receptors. Mol Cell Endocrinol. 2009. 310(1-2):3–10.43. Li BY, Tong J, Zhang ZL. [Exogenous estrogen improved calcium homeostasis and skeletal mineralization in vitamin D receptor gene knockout female mice.]. Sheng Li Xue Bao. 2006. 58(6):573–576.44. Venken K, Callewaert F, Boonen S, Vanderschueren D. Sex hormones, their receptors and bone health. Osteoporos Int. 2008. 19(11):1517–1525.45. Auld KL, Berasi SP, Liu Y, Cain M, Zhang Y, Huard C, Fukayama S, Zhang J, Choe S, Zhong W, Bhat BM, Bhat RA, Brown EL, Martinez RV. Estrogen-related receptor α regulates osteoblast differentiation via Wnt/β-catenin signaling. J Mol Endocrinol. 2012. 48(2):177–191.46. Vico L, Vanacker JM. Sex hormones and their receptors in bone homeostasis: insights from genetically modified mouse models. Osteoporos Int. 2010. 21(3):365–372.47. Chilibeck PD, Cornish SM. Effect of estrogenic compounds (estrogen or phytoestrogens) combined with exercise on bone and muscle mass in older individuals. Appl Physiol Nutr Metab. 2008. 33(1):200–212.48. Syed FA, Fraser DG, Spelsberg TC, Rosen CJ, Krust A, Chambon P, Jameson JL, Khosla S. Effects of loss of classical estrogen response element signaling on bone in male mice. Endocrinology. 2007. 148(4):1902–1910.49. Knowlton AA, Lee AR. Estrogen and the cardiovascular system. Pharmacol Ther. 2012. 135(1):54–70.50. Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2008. 73(9-10):864–869.51. Luksha L, Kublickiene K. The role of estrogen receptor subtypes for vascular maintenance. Gynecol Endocrinol. 2009. 25(2):82–95.52. Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metab. 2011. 14(3):289–299.53. Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009. 150(5):2109–2117.54. Faulds MH, Zhao C, Dahlman-Wright K, Gustafsson JA. The diversity of sex steroid action: regulation of metabolism by estrogen signaling. J Endocrinol. 2012. 212(1):3–12.55. Kalita K, Szymczak S. [Estrogen receptors in the brain]. Neurol Neurochir Pol. 2003. 37:Suppl 3. 63–78.56. Hill RA, Boon WC. Estrogens, brain, and behavior: lessons from knockout mouse models. Semin Reprod Med. 2009. 27(3):218–228.57. Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009. 196(2):254–260.58. Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience. 2006. 138(3):921–928.59. Crews D, Fuller T, Mirasol EG, Pfaff DW, Ogawa S. Postnatal environment affects behavior of adult transgenic mice. Exp Biol Med (Maywood). 2004. 229(9):935–939.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Growth and Pubertal Development in Female Mice with Tissue-specific Knock out of Estrogen Receptor
- The Relationship Between the Expression of Estrogen Receptor beta and Recurrence in Breast Cancer
- Gender-specific Colorectal Cancer: Epidemiologic Difference and Role of Estrogen
- Role of Estrogen Receptor-alpha in the Regulation of Claudin-6 Expression in Breast Cancer Cells
- The Clinical Significance of the Estrogen Receptor beta Expression for Endocrine Therapy in Patients with ERalpha-negative and Progesterone Receptor-positive Breast Carcinoma