J Bacteriol Virol.
2012 Mar;42(1):41-47. 10.4167/jbv.2012.42.1.41.
Production of the Monoclonal Antibodies against Bartonella henselae Isolated from a Korean Patient
- Affiliations
-
- 1Department of Microbiology, Inha University School of Medicine, Incheon, Korea. jaeskang@inha.ac.kr
- KMID: 1434771
- DOI: http://doi.org/10.4167/jbv.2012.42.1.41
Abstract
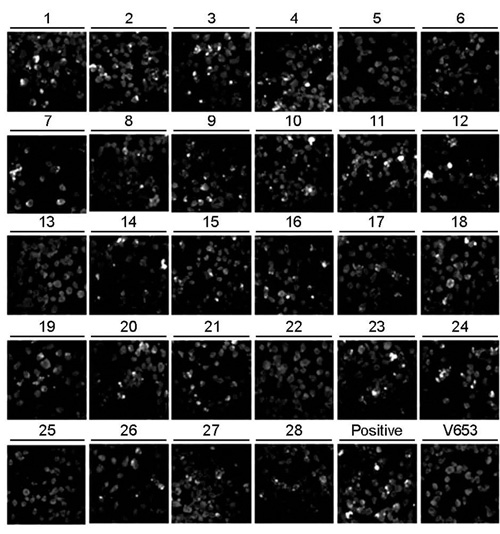
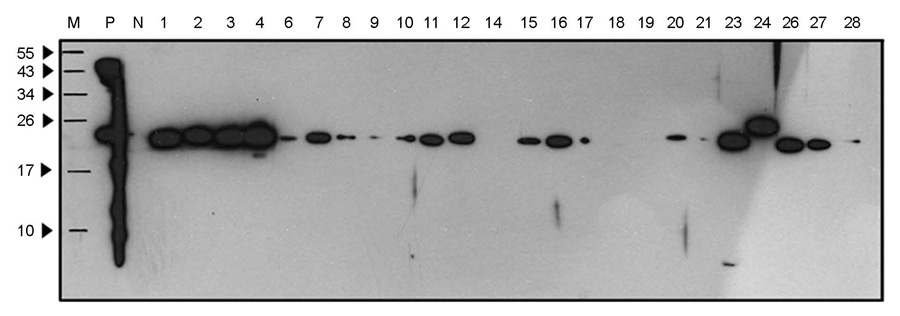
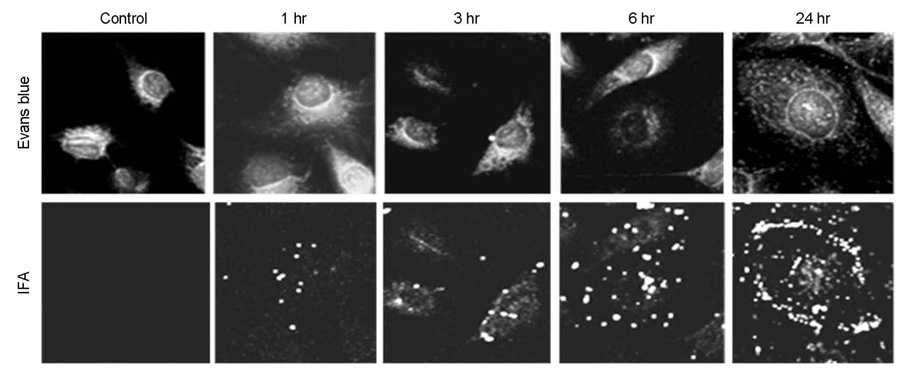
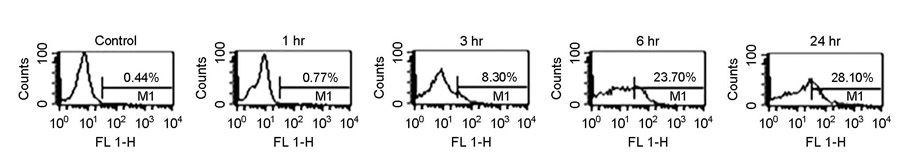
- Bartonellosis is spotlighted recently as an emerging zoonosis and Bartonella henselae is reported to be the main infectious agent. In Korea, however, few studies have been made on the epidemiology and microbiology on bartonellosis. Thus, this study was conducted to produce a new monoclonal antibody that can be used for identifying B. henselae. In order to prepare monoclonal antibodies against B. henselae, we inoculated mice with the isolated strain from Korean patient and performed cell fusion experiment. The selected hybridoma clones produced monoclonal antibodies which showed positive immunofluorescence staining of bacteria and specific protein bands in western blot analysis. In order to examine whether these antibodies could be used for the identifying and quantifying Bartonella, we performed confocal microscopy and flow cytometry using the new antibodies. These monoclonal antibodies can be used as a useful tool in further researches on the biology of Bartonella.
MeSH Terms
Figure
Cited by 1 articles
-
First Case of Bartonella henselae Bacteremia in Korea
Jae-Hyoung Im, Ji Hyeon Baek, Hyun-Jung Lee, Jin-Soo Lee, Moon-Hyun Chung, Mijeong Kim, Sun Myoung Lee, Jae-Seung Kang
Infect Chemother. 2013;45(4):446-450. doi: 10.3947/ic.2013.45.4.446.
Reference
-
1. Chomel BB, Abbott RC, Kasten RW, Floyd-Hawkins KA, Kass PH, Glaser CA, et al. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J Clin Microbiol. 1995. 33:2445–2450.
Article2. Koehler JE, Glaser CA, Tappero JW. Rhochalimaea henselae infection. A new zoonosis with the domestic cat as reservoir. JAMA. 1994. 271:531–535.
Article3. Garcia-Caceres U, Garcia FU. Bartonellosis, An immunodepressive disease and the life of Daniel Alcides Carrión. Am J Clin Pathol. 1991. 95:S58–S66.4. Schultz MG. Daniel Carrión's experiment. N Engl J Med. 1968. 278:1323–1326.
Article5. Ihler GM. Bartonella bacilliformis: dangerous pathogen slowly emerging from deep background. FEMS Microbiol Lett. 1996. 144:1–11.
Article6. Bass JW, Vincent JM, Person DA. The expanding spectrum of Bartonella infections: II. Cat-scratch disease. Pediatr infect Dis J. 1997. 16:163–179.7. Stoler MH, Bonfiglio TA, Steigbigel RT, Pereira M. An atypical subcutaneous infection associated with acquired immune deficiency syndrome. Am J Clin Pathol. 1983. 80:714–718.
Article8. Slater LN, Welch DF, Hensel D, Coody DW. A newly recognized fastidious gram-negative pathogen as a cause of fever and bacteremia. N Engl J Med. 1990. 323:1587–1593.
Article9. Welch DF, Pickett DA, Slater LN, Steigerwalt AG, Brenner DJ. Rochalimaea henselae sp. nov., a cause of septicemia, bacillary angiomatosis, and parenchymal bacillary peliosis. J Clin Microbiol. 1992. 30:275–280.
Article10. Birtles RJ, Harrison TG, Saunders NA, Molyneux DH. Proposals to unify the genera Grahamella and Bartonella, with descriptions of Bartonella talpae comb. nov., Bartonella peromysci comb. nov., and three new species, Bartonella grahamii sp. nov., Bartonella taylorii sp. nov., and Bartonella doshiae sp. nov. Int J Syst Bacteriol. 1995. 45:1–8.
Article11. Tsukahara M, Tsuneoka H, Iino H, Ohno K, Murano I. Bartonella henselae infection from a dog. Lancet. 1998. 352:1682.12. Bass JW, Vincent JM, Person DA. The expanding spectrum of Bartonella infections: I. Bartonellosis and trench fever. Pediatr Infect Dis J. 1997. 16:2–10.
Article13. Jackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993. 83:1707–1711.
Article14. Carithers HA. Cat-scratch disease. An overview based on a study of 1,200 patients. Am J Dis Child. 1985. 139:1124–1133.15. Maurin M, Raoult D. Bartonella infections: Diagnostic and management issues. Curr Opin Infect Dis. 1998. 11:189–193.16. Finkelstein JL, Brown TP, O'Reilly KL, Wedincamp J Jr, Foil LD. Studies on the growth of Bartonella henselae in the cat flea (Siphonaptera: Pulicidae). J Med Entomol. 2002. 39:915–919.
Article17. Agan BK, Dolan MJ. Laboratory diagnosis of Bartonella infections. Clin Lab Med. 2002. 22:937–962.18. Chung JY, Koo JW, Kim SW, Yoo YS, Han TH, Lim SJ. A case of cat scratch disease confirmed by polymerase chain reaction for Bartonella henselae DNA. Korean J Pediatr. 2005. 48:789–792.19. Chenoweth MR, Greene CE, Krause DC, Gherardini FC. Predominant outer membrane antigens of Bartonella henselae. Infect Immun. 2004. 72:3097–3105.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The seroprevalence of Bartonella henselae in healthy adults in Korea
- First Case of Bartonella henselae Bacteremia in Korea
- Molecular detection of Bartonella henselae DNA from fleas obtained from dogs, Korea
- A Case of Cat Scratch Disease Confirmed by Polymerase Chain Reaction for Bartonella henselae DNA
- Infections by pathogens with different transmission modes in feral cats from urban and rural areas of Korea