J Bacteriol Virol.
2012 Sep;42(3):224-231. 10.4167/jbv.2012.42.3.224.
Adenovirus Expressing Human Interferon Inhibits Replication of Foot and Mouth Disease Virus and Reduces Fatal Rate in Mice
- Affiliations
-
- 1Animal, Plant and Fisheries Quarantine and Inspection Agency (QIA), Anyang, Korea. parkjhvet@korea.kr
- 2Institute for Animal Health, Pirbright, Woking, Surrey, GU24 ONF, United Kingdom.
- KMID: 1434751
- DOI: http://doi.org/10.4167/jbv.2012.42.3.224
Abstract
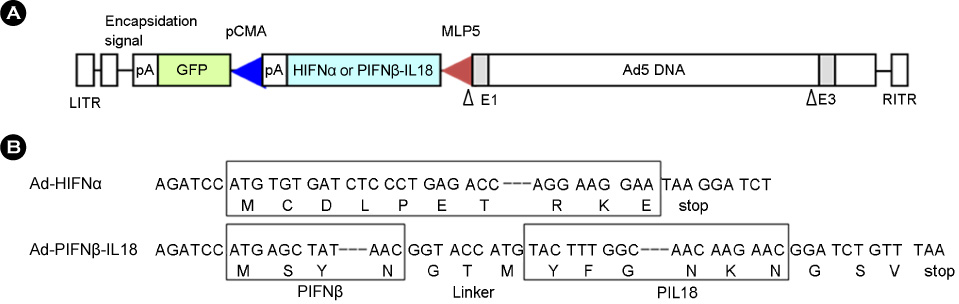
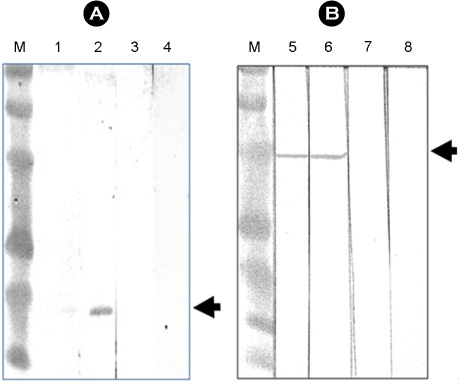
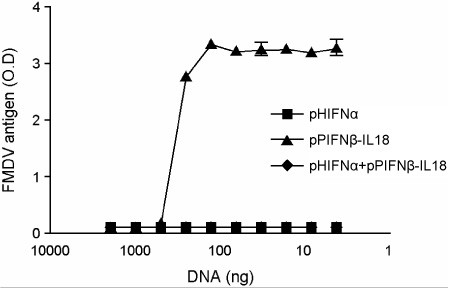
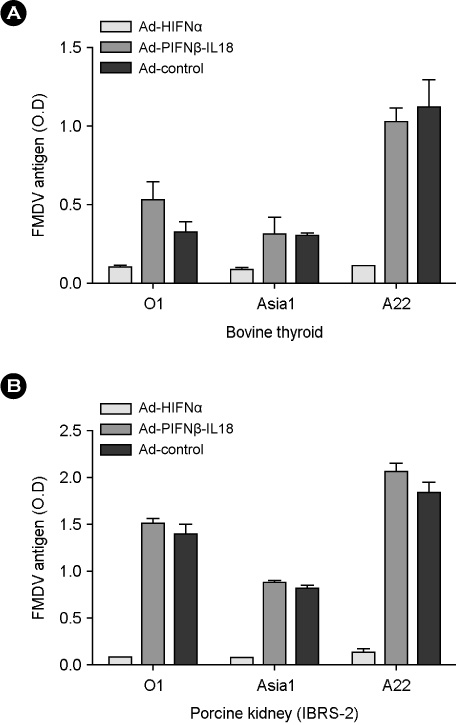
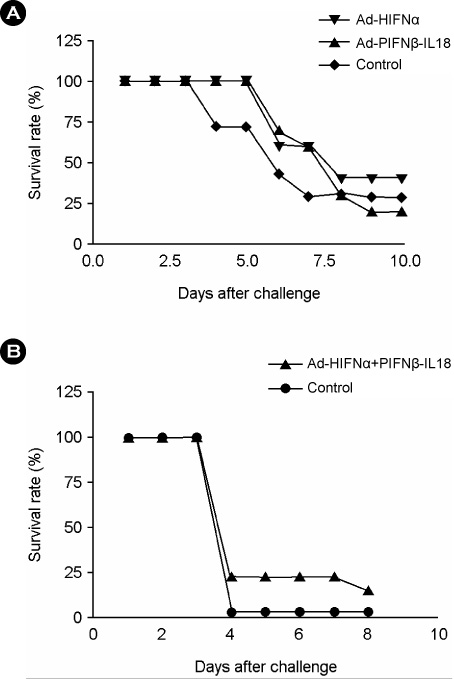
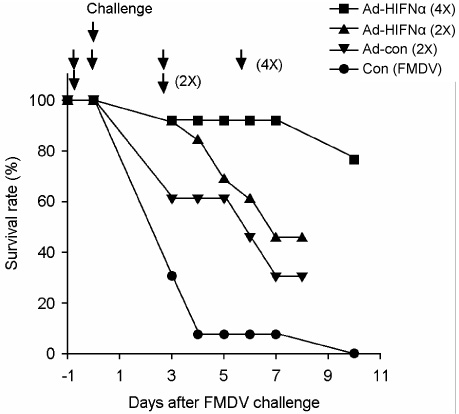
- Interferon is an important cytokine that plays a critical role in the initial host defense against viral infection. Recombinant human adenoviruses expressing human interferon-alpha (Ad-HIFNalpha) or pig interferon-beta fused with interleukin-18 (Ad-PIFNbeta-IL18) were constructed and used to induce an early protective response against foot and mouth disease (FMD). To analyze the antiviral effect, bovine thyroid and porcine kidney IBRS-2 cells and ICR mice were treated with Ad-HIFNalpha, Ad-PIFNbeta-IL18, and cocktail of Ad-HIFNalpha and Ad-PIFNbeta-IL18. The survival rate of suckling mice was monitored after foot and mouth disease virus (FMDV) challenge following intra-peritoneal (IP) administration of appropriate adenovirus. Indirect antigen ELISA was performed to evaluate inhibition of FMDV replication following challenge with the FMDV O, A, or Asia 1 serotypes in vitro. These recombinant adenoviruses reduced the replication of FMDV in susceptible cells, thereby decreasing the fatality in mice, suggesting that they can be a useful control method for the early protection against FMD infection in livestock after field trial.
Keyword
MeSH Terms
-
Adenoviridae
Adenoviruses, Human
Animals
Asia
Enzyme-Linked Immunosorbent Assay
Foot
Foot-and-Mouth Disease
Foot-and-Mouth Disease Virus
Humans
Interferon-alpha
Interferon-beta
Interferons
Interleukin-18
Kidney
Livestock
Mice
Mice, Inbred ICR
Survival Rate
Thyroid Gland
Interferon-alpha
Interferon-beta
Interferons
Interleukin-18
Figure
Reference
-
1. Bachrach HL. Foot-and-Mouth disease. Annu Rev Microbiol. 1968. 22:201–244.
Article2. Edward S. Manual of Dignostic Tests and Vaccines for Terrestrial. 2004. 5th ed. Paris: OIE;111–128.3. De los Santos T, Wu Q, De Avila Bottlon S, Grubman MJ. Short hairpin RNA targeted to the highly conserved 2B nonstructural protein coding region inhibits replication of multiple serotypes of foot-and-mouth disease virus. Virology. 2005. 335:222–231.
Article4. Moraes MP, Chinsangaram J, Brum MC, Grubman MJ. Immediate protection of swine from foot-and-mouth disease: a combination of adenoviruses expressing interferon alpha and a foot-and-mouth disease virus subunit vaccine. Vaccine. 2003. 22:268–279.
Article5. ShengFeng C, Ping L, Tao S, Xin W, Guo Feng W. Construction expression, purification, refold and activity assay of a specific scFv fragment against foot and mouth disease virus. Vet Res Commun. 2003. 27:243–256.6. Zhou A, Paranjape JM, Der SD, Wiliams BR, Silverman RH. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology. 1999. 258:435–440.
Article7. Stark GR, Kerr IM, Wiliams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998. 67:227–264.
Article8. Fournout S, Dozois CM, Yerle M, Pinton P, Fairbrother JM, Oswald E, et al. Cloning, chromosomal location, and tissue expression of the gene for pig interleukin-18. Immunogenetics. 2000. 51:358–365.
Article9. Qin XQ, Tao N, Dergay A, Moy P, Fawell S, Davis A, et al. Interferon-beta gene therapy inhibits tumor formation and causes regression of established tumors in immune-deficient mice. Proc Natl Acad Sci U S A. 1998. 95:14411–14416.
Article10. Ertl HC, Xiang Z. Novel vaccine approaches. J Immunol. 1996. 156:3579–3582.11. Hsu KH, Lubeck MD, Bhat BM, Bhat RA, Kostek B, Selling BH, et al. Efficacy of adenovirus-vectored respiratory syncytial virus vaccines in a new ferret model. Vaccine. 1994. 12:607–612.
Article12. Mittal SK, McDermott MR, Johnson DC, Prevec L, Graham FL. Monitoring foreign gene expression by a human adenovirus-based vector using the firefly luciferase gene as a reporter. Virus Res. 1993. 28:67–90.
Article13. Gogev S, Georgin JP, Schynts F, Vanderplasschen A, Thiry E. Bovine herpesvirus 1 glycoprotein D expression in bovine upper respiratory tract mediated by a human adenovirus type 5. Vet Res. 2004. 35:715–721.
Article14. Zhao J, Lou Y, Pinczewski J, Malkevitch N, Aldrich K, Kalyanaraman VS, et al. Boosting of SIV-specific immune responses in rhesus macaques by repeated administration of Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinants. Vaccine. 2003. 21:4022–4035.
Article15. Prevec L, Schneider M, Rosenthal KL, Belbeck LW, Derbyshire JB, Graham FL. Use of human adenovirus-based vectors for antigen expression in animals. J Gen Virol. 1989. 70:429–434.
Article16. Chinsangaram J, Moraes MP, Koster M, Grubman MJ. Novel viral disease control strategy: adenovirus expressing alpha interferon rapidly protects swine from foot-and-mouth disease. J Virol. 2003. 77:1621–1625.
Article17. Wu Q, Brum MC, Caron L, Koster M, Grubman MJ. Adenovirus-mediated type I interferon expression delays and reduces disease signs in cattle challenged with foot-and-mouth disease virus. J Interferon Cytokine Res. 2003. 23:359–368.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synthetic and Adenovirus Delivered Small Interference RNA Pools Targeting Conserved Regions of Foot-and-Mouth Disease Virus
- Development of a packaging cell line for propagation of replication-deficient adenovirus vector
- Establishment and evaluation of a murine alphavbeta3-integrin-expressing cell line with increased susceptibility to Foot-and-mouth disease virus
- Isolation of Echovirus Serotype 25 from Patient with Hand , Foot and Mouth Disease in Pusan , 1998
- Polarization of protective immunity induced by replication-incompetent adenovirus expressing glycoproteins of pseudorabies virus