Korean J Physiol Pharmacol.
2013 Feb;17(1):99-109. 10.4196/kjpp.2013.17.1.99.
Influence of Fimasartan (a Novel AT1 Receptor Blocker) on Catecholamine Release in the Adrenal Medulla of Spontaneously Hypertensive Rats
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Seoul National University, Seoul 710-744, Korea.
- 2Department of Chest Surgery, College of Medicine, Chosun University, Gwangju 501-759, Korea.
- 3Department of Pharmacology, College of Medicine, Chosun University, Gwangju 501-759, Korea. dylim@chosun.ac.kr
- KMID: 1432769
- DOI: http://doi.org/10.4196/kjpp.2013.17.1.99
Abstract
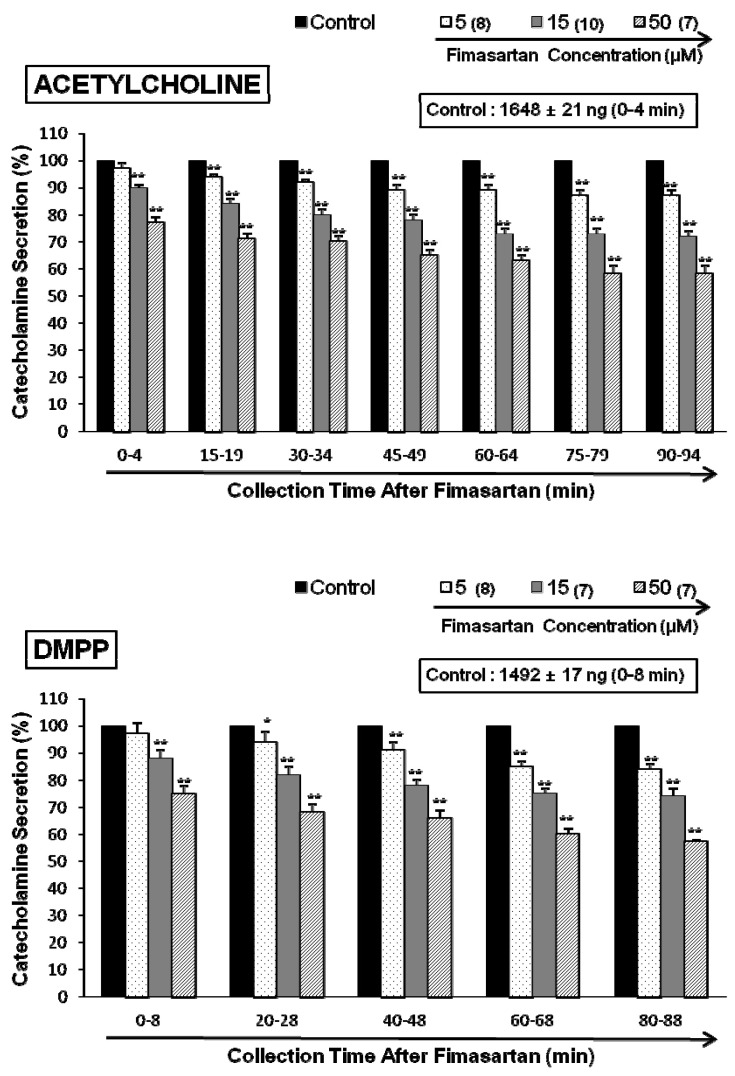
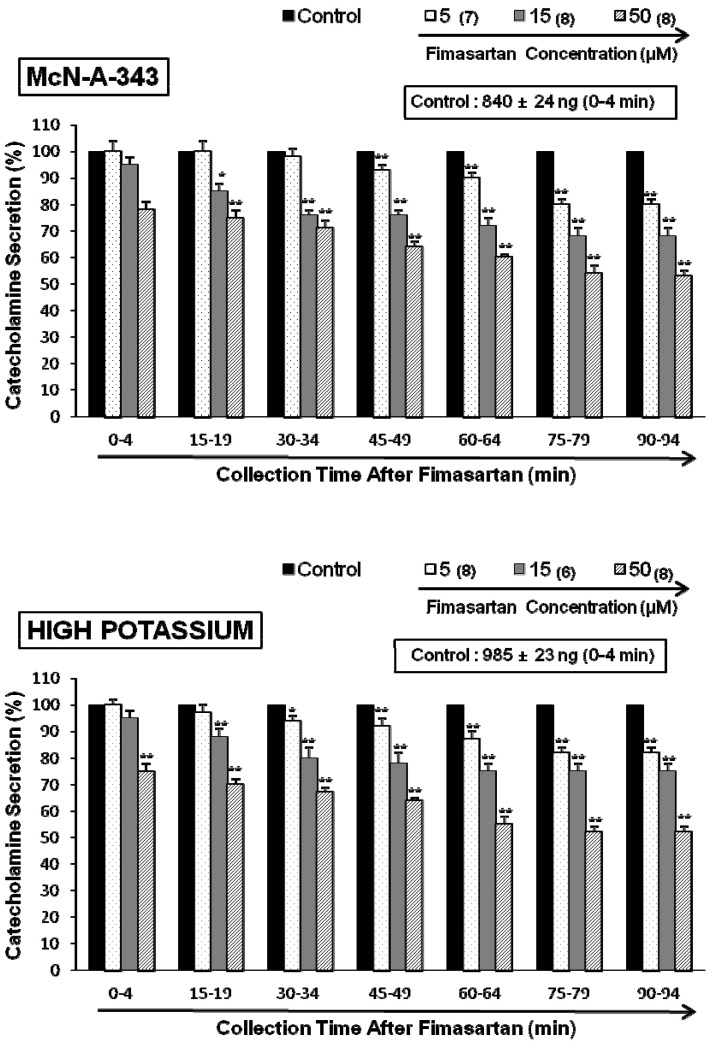
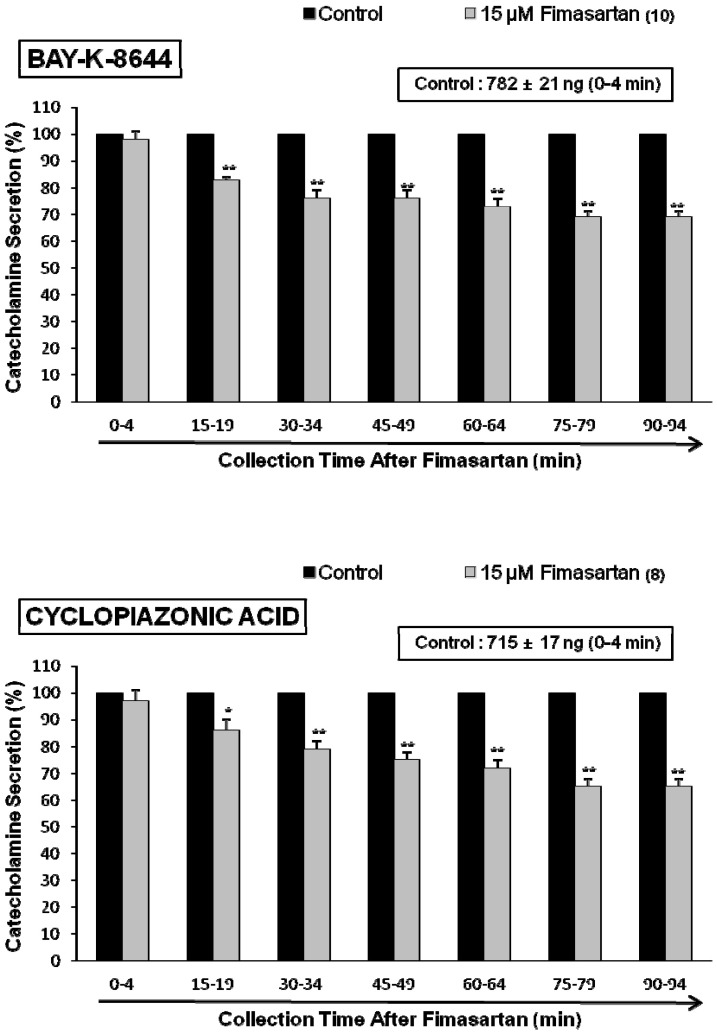
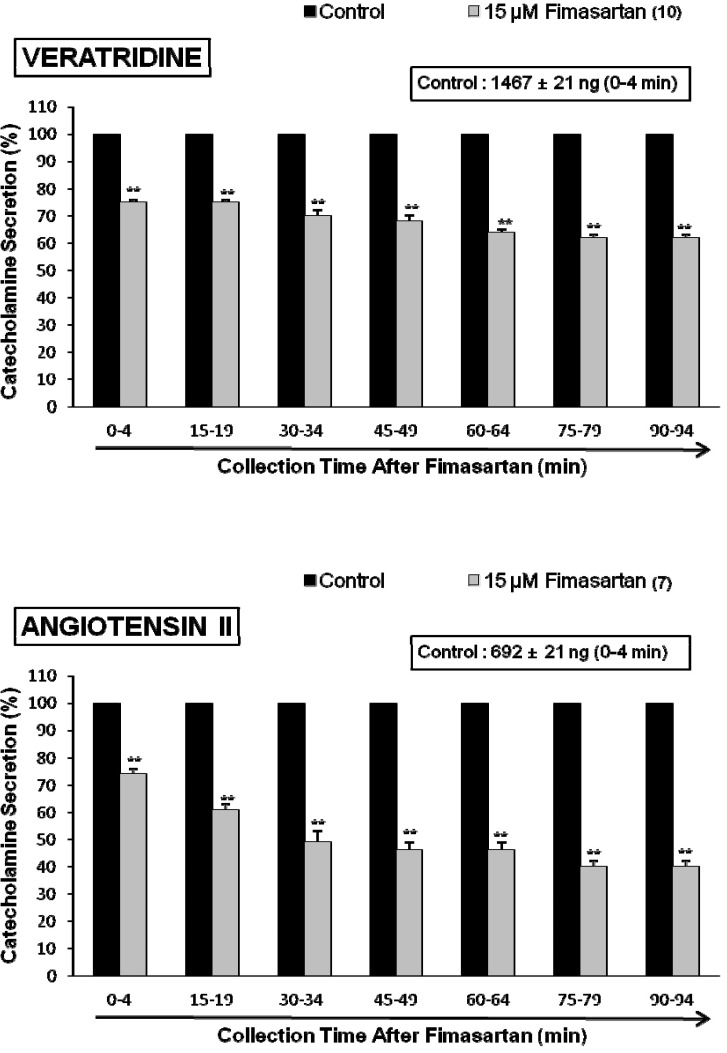
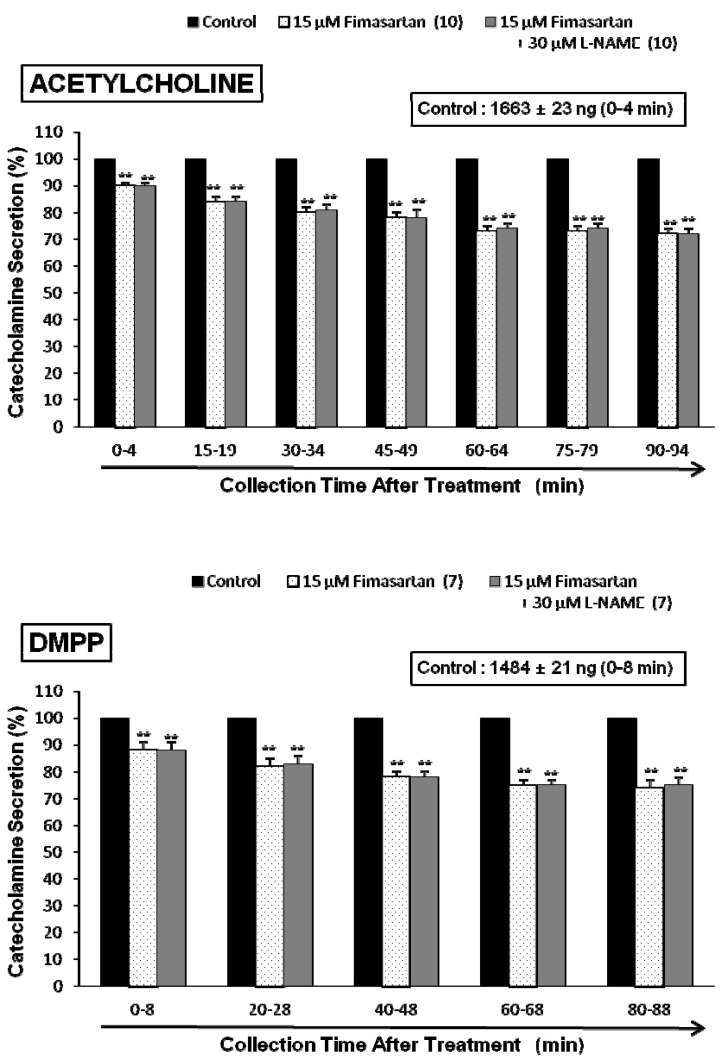
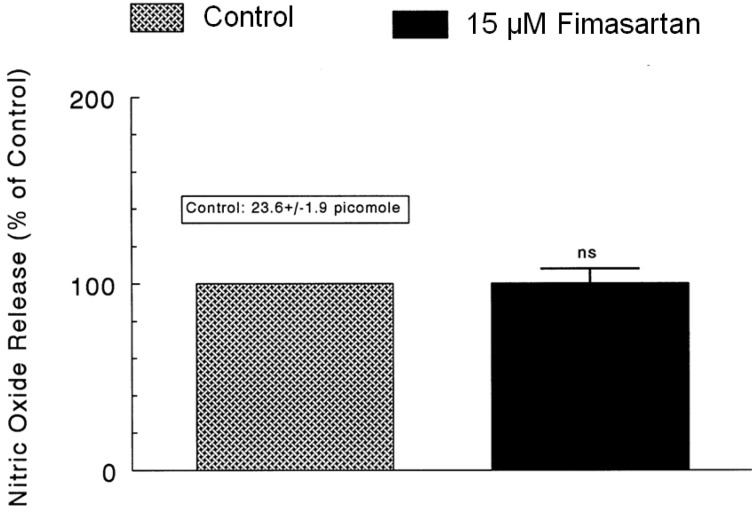
- The aim of this study was to determine whether fimasartan, a newly developed AT1 receptor blocker, can affect the CA release in the isolated perfused model of the adrenal medulla of spontaneously hypertensive rats (SHRs). Fimasartan (5~50 microM) perfused into an adrenal vein for 90 min produced dose- and time-dependently inhibited the CA secretory responses evoked by ACh (5.32 mM), high K+ (56 mM, a direct membrane depolarizer), DMPP (100 microM) and McN-A-343 (100 microM). Fimasartan failed to affect basal CA output. Furthermore, in adrenal glands loaded with fimasartan (15 microM), the CA secretory responses evoked by Bay-K-8644 (10 microM, an activator of L-type Ca2+ channels), cyclopiazonic acid (10 microM, an inhibitor of cytoplasmic Ca(2+)-ATPase), and veratridine (100 microM, an activator of Na+ channels) as well as by angiotensin II (Ang II, 100 nM), were markedly inhibited. In simultaneous presence of fimasartan (15 microM) and L-NAME (30 microM, an inhibitor of NO synthase), the CA secretory responses evoked by ACh, high K+, DMPP, Ang II, Bay-K-8644, and veratridine was not affected in comparison of data obtained from treatment with fimasartan (15 microM) alone. Also there was no difference in NO release between before and after treatment with fimasartan (15 microM). Collectively, these experimental results suggest that fimasartan inhibits the CA secretion evoked by Ang II, and cholinergic stimulation (both nicotininc and muscarinic receptors) as well as by membrane depolarization from the rat adrenal medulla. It seems that this inhibitory effect of fimasartan may be mediated by blocking the influx of both Na+ and Ca2+ through their ion channels into the rat adrenomedullary chromaffin cells as well as by inhibiting the Ca2+ release from the cytoplasmic calcium store, which is relevant to AT1 receptor blockade without NO release.
MeSH Terms
-
(4-(m-Chlorophenylcarbamoyloxy)-2-butynyl)trimethylammonium Chloride
3-Pyridinecarboxylic acid, 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-, Methyl ester
Adrenal Glands
Adrenal Medulla
Angiotensin II
Animals
Biphenyl Compounds
Calcium
Chromaffin Cells
Cytoplasm
Dimethylphenylpiperazinium Iodide
Indoles
Ion Channels
Membranes
NG-Nitroarginine Methyl Ester
Pyrimidines
Rats
Rats, Inbred SHR
Tetrazoles
Veins
Veratridine
(4-(m-Chlorophenylcarbamoyloxy)-2-butynyl)trimethylammonium Chloride
3-Pyridinecarboxylic acid, 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-, Methyl ester
Angiotensin II
Biphenyl Compounds
Calcium
Dimethylphenylpiperazinium Iodide
Indoles
Ion Channels
NG-Nitroarginine Methyl Ester
Pyrimidines
Tetrazoles
Veratridine
Figure
Reference
-
1. Kim JH, Lee JH, Paik SH, Kim JH, Chi YH. Fimasartan, a novel angiotensin II receptor antagonist. Arch Pharm Res. 2012; 35:1123–1126. PMID: 22864732.
Article2. Miura S, Karnik SS, Saku K. Review: angiotensin II type 1 receptor blockers: class effects versus molecular effects. J Renin Angiotensin Aldosterone Syst. 2011; 12:1–7. PMID: 20603272.
Article3. Böhler S, Pittrow D, Bramlage P, Kirch W. Drug interactions with angiotensin receptor blockers. Expert Opin Drug Saf. 2005; 4:7–18. PMID: 15709894.
Article4. Redon J, Fabia MJ. Efficacy in angiotensin receptor blockade: a comparative review of data with olmesartan. J Renin Angiotensin Aldosterone Syst. 2009; 10:147–156. PMID: 19651759.
Article5. Yi S, Kim TE, Yoon SH, Cho JY, Shin SG, Jang IJ, Yu KS. Pharmacokinetic interaction of fimasartan, a new angiotensin II receptor antagonist, with amlodipine in healthy volunteers. J Cardiovasc Pharmacol. 2011; 57:682–689. PMID: 21394036.
Article6. Shin KH, Kim TE, Kim SE, Lee MG, Song IS, Yoon SH, Cho JY, Jang IJ, Shin SG, Yu KS. The effect of the newly developed angiotensin receptor II antagonist fimasartan on the pharmacokinetics of atorvastatin in relation to OATP1B1 in healthy male volunteers. J Cardiovasc Pharmacol. 2011; 58:492–499. PMID: 21765368.
Article7. Lee SE, Kim YJ, Lee HY, Yang HM, Park CG, Kim JJ, Kim SK, Rhee MY, Oh BH. Investigators. Efficacy and tolerability of fimasartan, a new angiotensin receptor blocker, compared with losartan (50/100 mg): a 12-week, phase III, multicenter, prospective, randomized, double-blind, parallel-group, dose escalation clinical trial with an optional 12-week extension phase in adult Korean patients with mild-to-moderate hypertension. Clin Ther. 2012; 34:552–568. PMID: 22381711.
Article8. Oparil S, Silfani TN, Walker JF. Role of angiotensin receptor blockers as monotherapy in reaching blood pressure goals. Am J Hypertens. 2005; 18:287–294. PMID: 15752958.
Article9. Smith DH. Strategies to meet lower blood pressure goals with a new standard in angiotensin II receptor blockade. Am J Hypertens. 2002; 15:108S–114S. PMID: 12383591.
Article10. Teschemacher AG, Seward EP. Bidirectional modulation of exocytosis by angiotensin II involves multiple G-protein-regulated transduction pathways in chromaffin cells. J Neurosci. 2000; 20:4776–4785. PMID: 10864935.
Article11. Uresin Y, Erbas B, Ozek M, Ozkök E, Gürol AO. Losartan may prevent the elevation of plasma glucose, corticosterone and catecholamine levels induced by chronic stress. J Renin Angiotensin Aldosterone Syst. 2004; 5:93–96. PMID: 15295722.
Article12. Noh HJ, Kang YS, Lim DY. Effects of losartan on catecholamine release in the isolated rat adrenal gland. Korean J Physiol Pharmacol. 2009; 13:327–335. PMID: 19885018.
Article13. Lim HJ, Kim SY, Lim DY. Inhibitory effects of olmesartan on catecholamine secretion from the perfused rat adrenal medulla. Korean J Physiol Pharmacol. 2010; 14:241–248. PMID: 20827339.
Article14. Seltzer A, Bregonzio C, Armando I, Baiardi G, Saavedra JM. Oral administration of an AT1 receptor antagonist prevents the central effects of angiotensin II in spontaneously hypertensive rats. Brain Res. 2004; 1028:9–18. PMID: 15518636.
Article15. Critchley L, Ding B, Fok B, Wang D, Tomlinson B, James A, Thomas GN, Critchley J. The effects of candesartan and ramipril on adrenal catecholamine release in anaesthetized dogs. Eur J Pharmacol. 2004; 489:67–75. PMID: 15063157.
Article16. Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989; 14:177–183. PMID: 2759678.
Article17. Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000; 13:99S–105S. PMID: 10921528.18. Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension. 1999; 34:724–728. PMID: 10523349.
Article19. Cabassi A, Vinci S, Calzolari M, Bruschi G, Borghetti A. Regional sympathetic activity in pre-hypertensive phase of spontaneously hypertensive rats. Life Sci. 1998; 62:1111–1118. PMID: 9519813.
Article20. Trippodo NC, Frohlich ED. Similarities of genetic (spontaneous) hypertension. Man and rat. Circ Res. 1981; 48:309–319. PMID: 7460205.
Article21. Korner P, Bobik A, Oddie C, Friberg P. Sympathoadrenal system is critical for structural changes in genetic hypertension. Hypertension. 1993; 22:243–252. PMID: 8340160.
Article22. Lee RM, Borkowski KR, Leenen FH, Tsoporis J, Coughlin M. Combined effect of neonatal sympathectomy and adrenal demedullation on blood pressure and vascular changes in spontaneously hypertensive rats. Circ Res. 1991; 69:714–721. PMID: 1873866.
Article23. Borkowski KR, Quinn P. The effect of bilateral adrenal demedullation on vascular reactivity and blood pressure in spontaneously hypertensive rats. Br J Pharmacol. 1983; 80:429–437. PMID: 6640199.
Article24. Borkowski KR, Quinn P. Adrenaline and the development of spontaneous hypertension in rats. J Auton Pharmacol. 1985; 5:89–100. PMID: 2862149.
Article25. Borkowski KR. Effect of adrenal demedullation and adrenaline on hypertension development and vascular reactivity in young spontaneously hypertensive rats. J Auton Pharmacol. 1991; 11:1–14. PMID: 2030106.
Article26. Wakade AR. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J Physiol. 1981; 313:463–480. PMID: 7277230.
Article27. Anton AH, Sayre DF. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962; 138:360–375. PMID: 14013351.28. Tallarida RJ, Murray RB. Manual of pharmacologic calculations with computer programs. 1987. 2nd ed. New York: Springer-Verlag;p. 132.29. Hammer R, Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 1982; 31:2991–2998. PMID: 6897666.
Article30. Fulop T, Smith C. Matching native electrical stimulation by graded chemical stimulation in isolated mouse adrenal chromaffin cells. J Neurosci Methods. 2007; 166:195–202. PMID: 17714791.
Article31. García AG, Sala F, Reig JA, Viniegra S, Frías J, Fontériz R, Gandía L. Dihydropyridine BAY-K-8644 activates chromaffin cell calcium channels. Nature. 1984; 309:69–71. PMID: 6201747.
Article32. Lim DY, Kim CD, Ahn GW. Influence of TMB-8 on secretion of catecholamines from the perfused rat adrenal glands. Arch Pharm Res. 1992; 15:115–125.
Article33. Goeger DE, Riley RT. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 1989; 38:3995–4003. PMID: 2532015.34. Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989; 264:17816–17823. PMID: 2530215.35. Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000; 26:13–25. PMID: 10798388.36. Wada A, Takara H, Izumi F, Kobayashi H, Yanagihara N. Influx of 22Na through acetylcholine receptor-associated Na channels: relationship between 22Na influx, 45Ca influx and secretion of catecholamines in cultured bovine adrenal medulla cells. Neuroscience. 1985; 15:283–292. PMID: 2409474.37. Hano T, Mizukoshi M, Baba A, Nakamura N, Nishio I. Angiotensin II subtype 1 receptor modulates epinephrine release from isolated rat adrenal gland. Blood Press Suppl. 1994; 5:105–108. PMID: 7889190.38. Bader MF, Holz RW, Kumakura K, Vitale N. Exocytosis: the chromaffin cell as a model system. Ann N Y Acad Sci. 2002; 971:178–183. PMID: 12438117.
Article39. Currie KP. Inhibition of Ca2+ channels and adrenal catecholamine release by G protein coupled receptors. Cell Mol Neurobiol. 2010; 30:1201–1208. PMID: 21061161.40. Borges R, Camacho M, Gillis KD. Measuring secretion in chromaffin cells using electrophysiological and electrochemical methods. Acta Physiol (Oxf). 2008; 192:173–184. PMID: 18021323.
Article41. Viveros OH, Wilson SP. The adrenal chromaffin cell as a model to study the co-secretion of enkephalins and catecholamines. J Auton Nerv Syst. 1983; 7:41–58. PMID: 6302158.
Article42. Livett BG, Marley PD. Noncholinergic control of adrenal catecholamine secretion. J Anat. 1993; 183:277–289. PMID: 7507911.43. Plunkett LM, Correa FM, Saavedra JM. Quantitative autoradiographic determination of angiotensin-converting enzyme binding in rat pituitary and adrenal glands with 125I-351A, a specific inhibitor. Regul Pept. 1985; 12:263–272. PMID: 3003818.
Article44. Phillips MI, Speakman EA, Kimura B. Levels of angiotensin and molecular biology of the tissue renin angiotensin systems. Regul Pept. 1993; 43:1–20. PMID: 8426906.45. Israel A, Strömberg C, Tsutsumi K, Garrido MR, Torres M, Saavedra JM. Angiotensin II receptor subtypes and phosphoinositide hydrolysis in rat adrenal medulla. Brain Res Bull. 1995; 38:441–446. PMID: 8665267.
Article46. Wong PC, Hart SD, Zaspel AM, Chiu AT, Ardecky RJ, Smith RD, Timmermans PB. Functional studies of nonpeptide angiotensin II receptor subtype-specific ligands: DuP 753 (AII-1) and PD123177 (AII-2). J Pharmacol Exp Ther. 1990; 255:584–592. PMID: 2243344.47. Armando I, Carranza A, Nishimura Y, Hoe KL, Barontini M, Terrón JA, Falcón-Neri A, Ito T, Juorio AV, Saavedra JM. Peripheral administration of an angiotensin II AT1 receptorantagonist decreases the hypothalamic-pituitary-adrenal response to isolation stress. Endocrinology. 2001; 142:3880–3889. PMID: 11517166.48. Yang G, Xi ZX, Wan Y, Wang H, Bi G. Changes in circulating and tissue angiotensin II during acute and chronic stress. Biol Signals. 1993; 2:166–172. PMID: 8004155.
Article49. Mizuno M, Sada T, Ikeda M, Fukuda N, Miyamoto M, Yanagisawa H, Koike H. Pharmacology of CS-866, a novel nonpeptide angiotensin II receptor antagonist. Eur J Pharmacol. 1995; 285:181–188. PMID: 8566137.
Article50. Marley PD, McLeod J, Anderson C, Thomson KA. Nerves containing nitric oxide synthase and their possible function in the control of catecholamine secretion in the bovine adrenal medulla. J Auton Nerv Syst. 1995; 54:184–194. PMID: 7490420.
Article51. Oset-Gasque MJ, Parramón M, Hortelano S, Boscá L, González MP. Nitric oxide implication in the control of neurosecretion by chromaffin cells. J Neurochem. 1994; 63:1693–1700. PMID: 7523598.
Article52. Palacios M, Knowles RG, Palmer RM, Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun. 1989; 165:802–809. PMID: 2480784.
Article53. Schwarz PM, Rodriguez-Pascual F, Koesling D, Torres M, Förstermann U. Functional coupling of nitric oxide synthase and soluble guanylyl cyclase in controlling catecholamine secretion from bovine chromaffin cells. Neuroscience. 1998; 82:255–265. PMID: 9483518.
Article54. Torres M, Ceballos G, Rubio R. Possible role of nitric oxide in catecholamine secretion by chromaffin cells in the presence and absence of cultured endothelial cells. J Neurochem. 1994; 63:988–996. PMID: 7519669.
Article55. Rodriguez-Pascual F, Miras-Portugal MT, Torres M. Effect of cyclic GMP-increasing agents nitric oxide and C-type natriuretic peptide on bovine chromaffin cell function: inhibitory role mediated by cyclic GMP-dependent protein kinase. Mol Pharmacol. 1996; 49:1058–1070. PMID: 8649344.56. Siragy HM, de Gasparo M, Carey RM. Angiotensin type 2 receptor mediates valsartan-induced hypotension in conscious rats. Hypertension. 2000; 35:1074–1077. PMID: 10818067.
Article57. Siragy HM, Carey RM. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension. 1999; 33:1237–1242. PMID: 10334818.58. Gross V, Milia AF, Plehm R, Inagami T, Luft FC. Long-term blood pressure telemetry in AT2 receptor-disrupted mice. J Hypertens. 2000; 18:955–961. PMID: 10930194.
Article59. Pueyo ME, Arnal JF, Rami J, Michel JB. Angiotensin II stimulates the production of NO and peroxynitrite in endothelial cells. Am J Physiol. 1998; 274:C214–C220. PMID: 9458730.60. Boulanger CM, Caputo L, Lévy BI. Endothelial AT1-mediated release of nitric oxide decreases angiotensin II contractions in rat carotid artery. Hypertension. 1995; 26:752–757. PMID: 7591014.61. Thorup C, Kornfeld M, Goligorsky MS, Moore LC. AT1 receptor inhibition blunts angiotensin II-stimulated nitric oxide release in renal arteries. J Am Soc Nephrol. 1999; 10(Suppl 11):S220–S224. PMID: 9892167.62. McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995; 57:521–546. PMID: 7778876.
Article63. Takekoshi K, Ishii K, Kawakami Y, Isobe K, Nakai T. Activation of angiotensin II subtype 2 receptor induces catecholamine release in an extracellular Ca2+-dependent manner through a decrease of cyclic guanosine 3',5'-monophosphate production in cultured porcine adrenal medullary chromaffin Cells. Endocrinology. 2001; 142:3075–3086. PMID: 11416030.64. Cheek TR, O'Sullivan AJ, Moreton RB, Berridge MJ, Burgoyne RD. Spatial localization of the stimulus-induced rise in cytosolic Ca2+ in bovine adrenal chromaffin cells. Distinct nicotinic and muscarinic patterns. FEBS Lett. 1989; 247:429–434. PMID: 2653866.65. Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995; 268:239–247. PMID: 7716515.
Article66. Holz RW, Senter RA, Frye RA. Relationship between Ca2+ uptake and catecholamine secretion in primary dissociated cultures of adrenal medulla. J Neurochem. 1982; 39:635–646. PMID: 7097273.67. Suzuki M, Muraki K, Imaizumi Y, Watanabe M. Cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca2+-pump, reduces Ca2+-dependent K+ currents in guinea-pig smooth muscle cells. Br J Pharmacol. 1992; 107:134–140. PMID: 1330156.68. Challis RA, Jones JA, Owen PJ, Boarder MR. Changes in inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate mass accumulations in cultured adrenal chromaffin cells in response to bradykinin and histamine. J Neurochem. 1991; 56:1083–1086. PMID: 1993889.
Article69. Dendorfer A, Raasch W, Tempel K, Dominiak P. Interactions between the renin-angiotensin system (RAS) and the sympathetic system. Basic Res Cardiol. 1998; 93(Suppl 2):24–29. PMID: 9833158.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Losartan on Catecholamine Release in the Isolated Rat Adrenal Gland
- Inhibitory Effects of Olmesartan on Catecholamine Secretion from the Perfused Rat Adrenal Medulla
- Differential brain angiotensin-II type I receptor expression in hypertensive rats
- ACE2 and Angiotensin-(1-7) in Hypertensive Renal Disease
- Gintonin facilitates catecholamine secretion from the perfused adrenal medulla