Korean J Physiol Pharmacol.
2013 Feb;17(1):89-97. 10.4196/kjpp.2013.17.1.89.
Differential Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on Motor Behavior and Dopamine Levels at Brain Regions in Three Different Mouse Strains
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Dankook University, Cheonan 330-714, Korea. hrkim@dankook.ac.kr
- KMID: 1432768
- DOI: http://doi.org/10.4196/kjpp.2013.17.1.89
Abstract
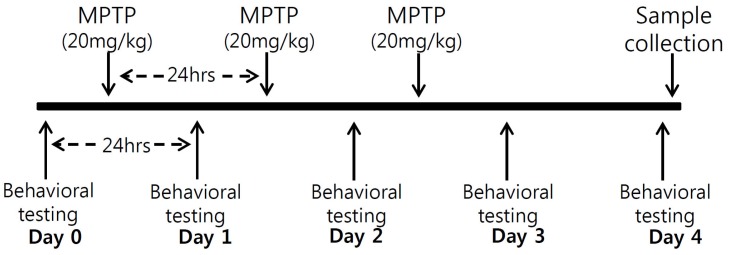
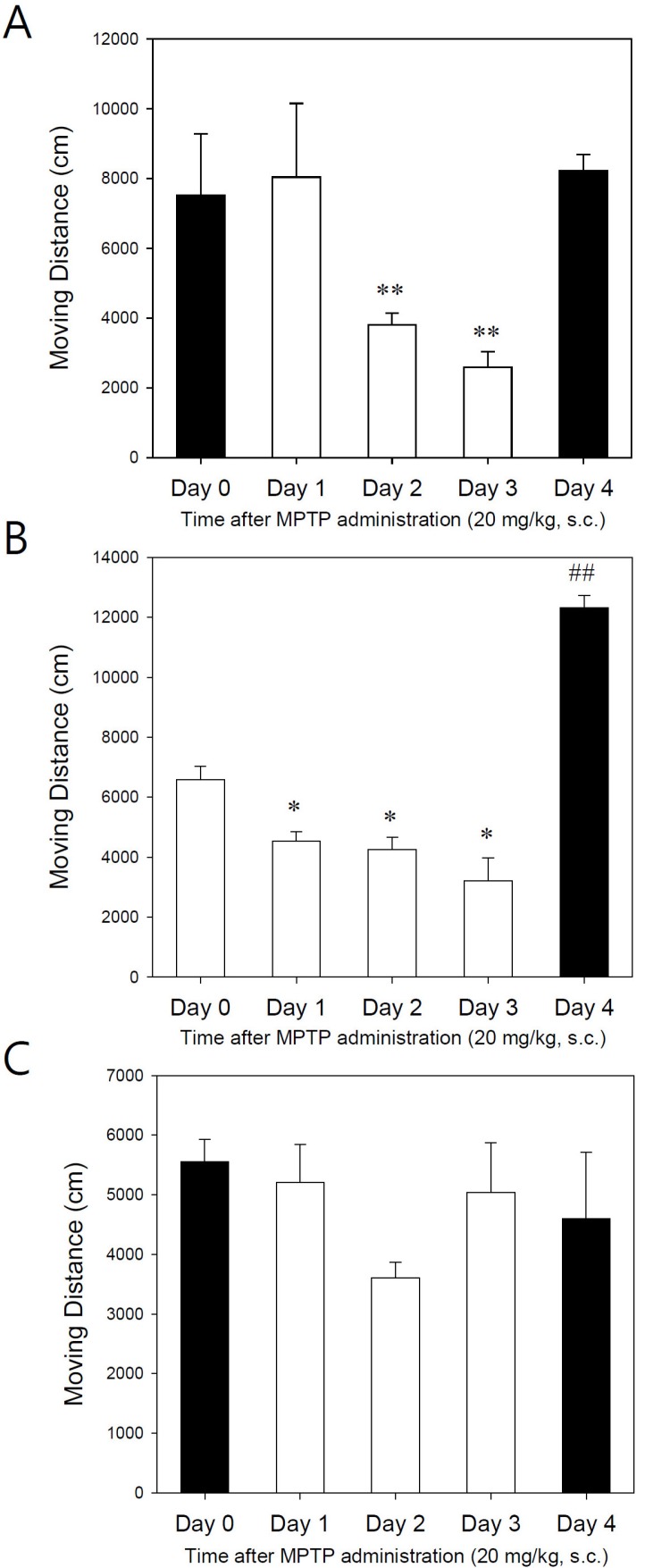
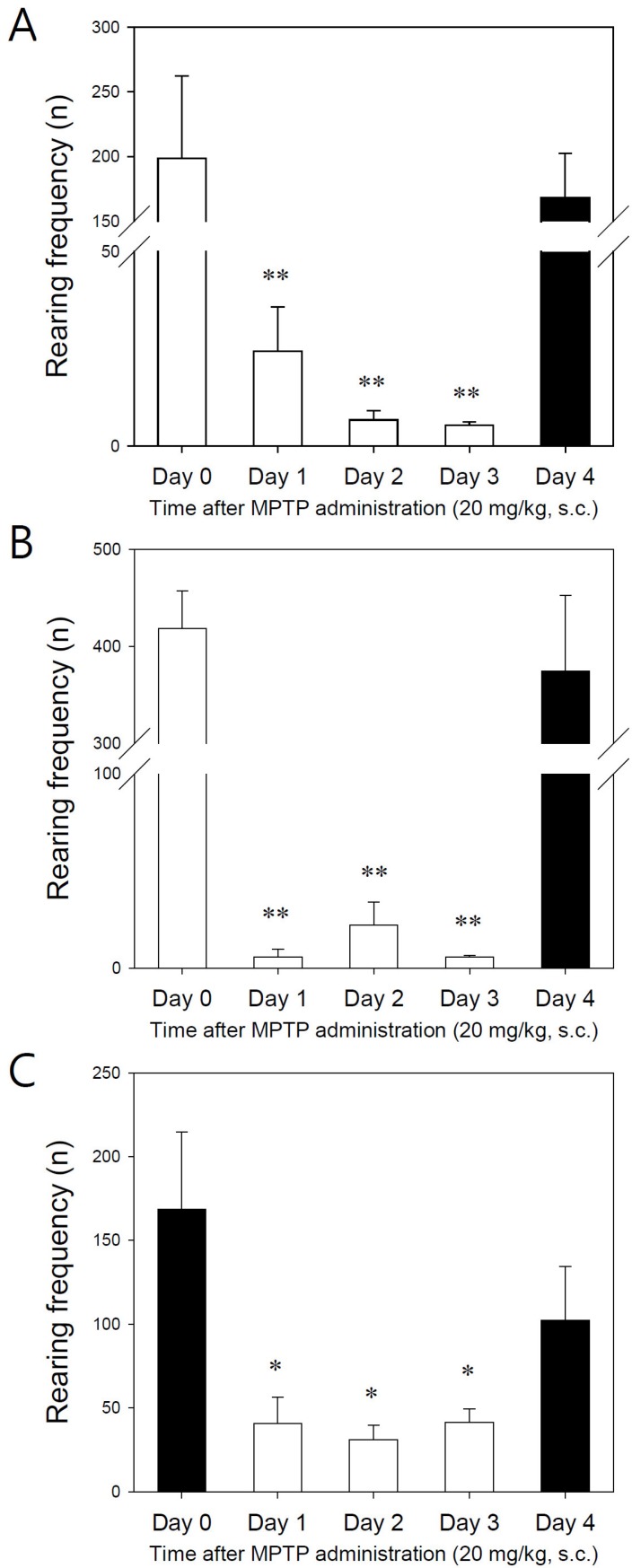
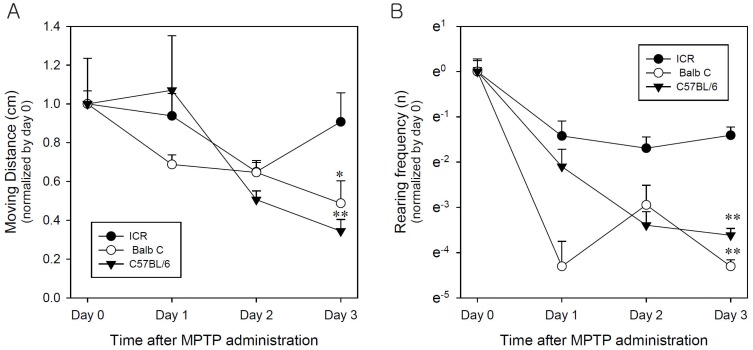
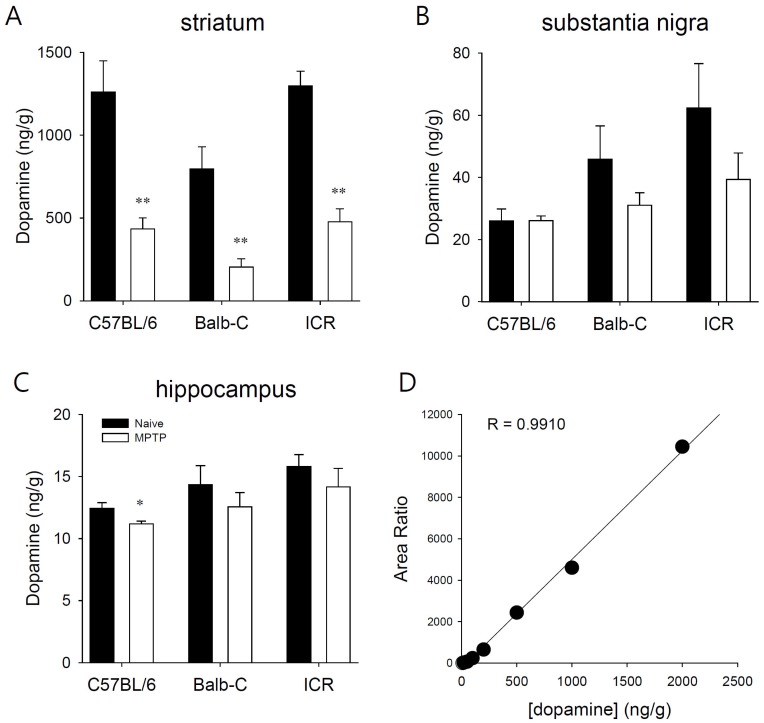
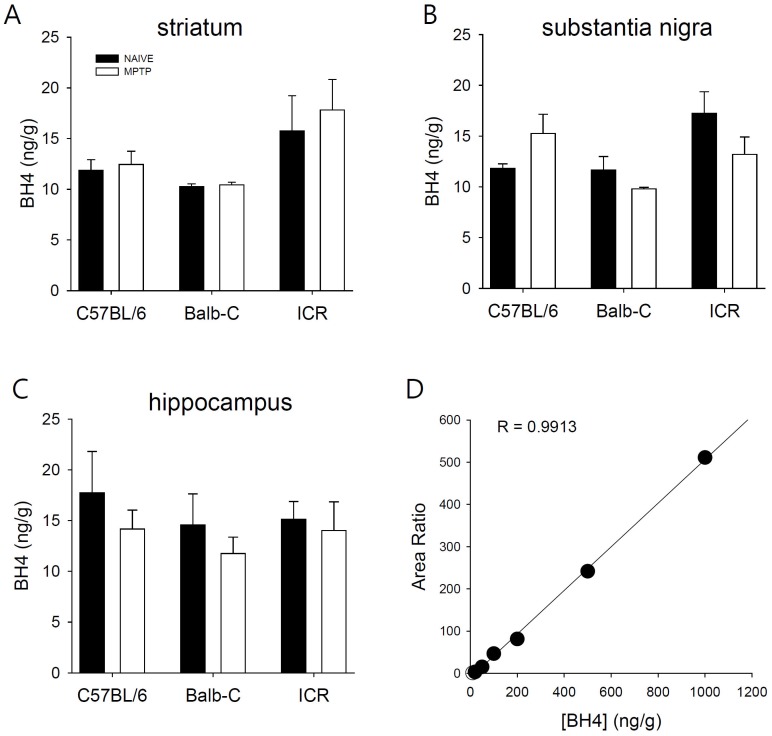
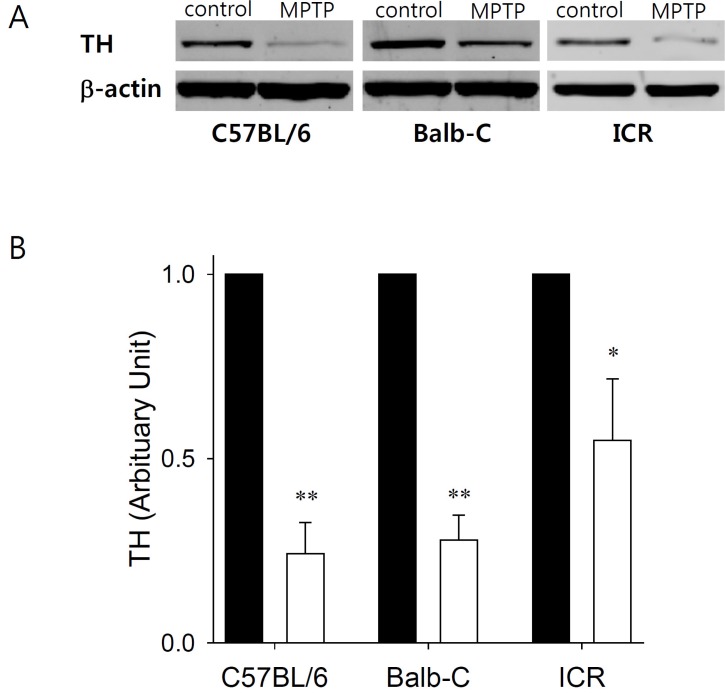
- Developing an animal model for a specific disease is very important in the understanding of the underlying mechanism of the disease and allows testing of newly developed new drugs before human application. However, which of the plethora of experimental animal species to use in model development can be perplexing. Administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a very well known method to induce the symptoms of Parkinson's disease in mice. But, there is very limited information about the different sensitivities to MPTP among mouse strains. Here, we tested three different mouse strains (C57BL/6, Balb-C, and ICR) as a Parkinsonian model by repeated MPTP injections. In addition to behavioral analysis, endogenous levels of dopamine and tetrahydrobiopterin in mice brain regions, such as striatum, substantia nigra, and hippocampus were directly quantified by liquid chromatography-tandem mass spectrometry. Repeated administrations of MPTP significantly affected the moving distances and rearing frequencies in all three mouse strains. The endogenous dopamine concentrations and expression levels of tyrosine hydroxylase were significantly decreased after the repeated injections, but tetrahydrobiopterin did not change in analyzed brain regions. However, susceptibilities of the mice to MPTP were differed based on the degree of behavioral change, dopamine concentration in brain regions, and expression levels of tyrosine hydroxylase, with C57BL/6 and Balb-C mice being more sensitive to the dopaminergic neuronal toxicity of MPTP than ICR mice.
Keyword
MeSH Terms
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Animals
Biopterin
Brain
Dopamine
Dopaminergic Neurons
Hippocampus
Humans
Mass Spectrometry
Mice
Mice, Inbred ICR
Models, Animal
Parkinson Disease
Substantia Nigra
Tyrosine 3-Monooxygenase
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
Biopterin
Dopamine
Tyrosine 3-Monooxygenase
Figure
Cited by 1 articles
-
Alteration of Striatal Tetrahydrobiopterin in Iron-Induced Unilateral Model of Parkinson's Disease
Bijay Aryal, Jin-Koo Lee, Hak Rim Kim, Hyung-Gun Kim
Korean J Physiol Pharmacol. 2014;18(2):129-134. doi: 10.4196/kjpp.2014.18.2.129.
Reference
-
1. Blandini F, Armentero MT. Animal models of Parkinson's disease. FEBS J. 2012; 279:1156–1166. PMID: 22251459.
Article2. Yokoyama H, Kuroiwa H, Kasahara J, Araki T. Neuropharmacological approach against MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced mouse model of Parkinson's disease. Acta Neurobiol Exp (Wars). 2011; 71:269–280. PMID: 21731080.3. Prediger RD, Aguiar AS Jr, Moreira EL, Matheus FC, Castro AA, Walz R, De Bem AF, Latini A, Tasca CI, Farina M, Raisman-Vozari R. The intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a new rodent model to test palliative and neuroprotective agents for Parkinson's disease. Curr Pharm Des. 2011; 17:489–507. PMID: 21375482.4. Onyango IG. Mitochondrial dysfunction and oxidative stress in Parkinson's disease. Neurochem Res. 2008; 33:589–597. PMID: 17940895.
Article5. Duty S, Jenner P. Animal models of Parkinson's disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011; 164:1357–1391. PMID: 21486284.
Article6. Giovanni A, Sieber BA, Heikkila RE, Sonsalla PK. Studies on species sensitivity to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Part 1: Systemic administration. J Pharmacol Exp Ther. 1994; 270:1000–1007. PMID: 7932147.7. Giovanni A, Sonsalla PK, Heikkila RE. Studies on species sensitivity to the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Part 2: Central administration of 1-methyl-4-phenylpyridinium. J Pharmacol Exp Ther. 1994; 270:1008–1014. PMID: 7932148.8. Ramsay RR, Singer TP. Energy-dependent uptake of N-methyl-4-phenylpyridinium, the neurotoxic metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, by mitochondria. J Biol Chem. 1986; 261:7585–7587. PMID: 3486869.
Article9. Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985; 36:2503–2508. PMID: 2861548.
Article10. Mizuno Y, Suzuki K, Sone N, Saitoh T. Inhibition of ATP synthesis by 1-methyl-4-phenylpyridinium ion (MPP+) in isolated mitochondria from mouse brains. Neurosci Lett. 1987; 81:204–208. PMID: 3501080.
Article11. Di Monte D, Jewell SA, Ekström G, Sandy MS, Smith MT. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 1-methyl-4-phenylpyridine (MPP+) cause rapid ATP depletion in isolated hepatocytes. Biochem Biophys Res Commun. 1986; 137:310–315. PMID: 3487319.
Article12. Volkmann J, Daniels C, Witt K. Neuropsychiatric effects of subthalamic neurostimulation in Parkinson disease. Nat Rev Neurol. 2010; 6:487–498. PMID: 20680036.
Article13. Voon V, Fernagut PO, Wickens J, Baunez C, Rodriguez M, Pavon N, Juncos JL, Obeso JA, Bezard E. Chronic dopaminergic stimulation in Parkinson's disease: from dyskinesias to impulse control disorders. Lancet Neurol. 2009; 8:1140–1149. PMID: 19909912.
Article14. Duch DS, Smith GK. Biosynthesis and function of tetrahydrobiopterin. J Nutr Biochem. 1991; 2:411–423.
Article15. Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond). 2007; 113:47–63. PMID: 17555404.
Article16. Sonsalla PK, Heikkila RE. Neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and methamphetamine in several strains of mice. Prog Neuropsychopharmacol Biol Psychiatry. 1988; 12:345–354. PMID: 3260386.
Article17. Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci USA. 1983; 80:4546–4550. PMID: 6192438.
Article18. Langston JW, Forno LS, Rebert CS, Irwin I. Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyrine (MPTP) in the squirrel monkey. Brain Res. 1984; 292:390–394. PMID: 6607092.19. Antzoulatos E, Jakowec MW, Petzinger GM, Wood RI. Sex differences in motor behavior in the MPTP mouse model of Parkinson's disease. Pharmacol Biochem Behav. 2010; 95:466–472. PMID: 20347863.
Article20. Kim HR, Kim TH, Hong SH, Kim HG. Direct detection of tetrahydrobiopterin (BH4) and dopamine in rat brain using liquid chromatography coupled electrospray tandem mass spectrometry. Biochem Biophys Res Commun. 2012; 419:632–637. PMID: 22382017.
Article21. Kim HR, Graceffa P, Ferron F, Gallant C, Boczkowska M, Dominguez R, Morgan KG. Actin polymerization in differentiated vascular smooth muscle cells requires vasodilator-stimulated phosphoprotein. Am J Physiol Cell Physiol. 2010; 298:C559–C571. PMID: 20018948.
Article22. Schwarting RK, Sedelis M, Hofele K, Auburger GW, Huston JP. Strain-dependent recovery of open-field behavior and striatal dopamine deficiency in the mouse MPTP model of Parkinson's disease. Neurotox Res. 1999; 1:41–56. PMID: 12835113.
Article23. Choi HJ, Lee SY, Cho Y, No H, Kim SW, Hwang O. Tetrahydrobiopterin causes mitochondrial dysfunction in dopaminergic cells: implications for Parkinson's disease. Neurochem Int. 2006; 48:255–262. PMID: 16343695.
Article24. Ichinose H, Nomura T, Sumi-Ichinose C. Metabolism of tetrahydrobiopterin: its relevance in monoaminergic neurons and neurological disorders. Chem Rec. 2008; 8:378–385. PMID: 19107867.
Article25. Foxton RH, Land JM, Heales SJ. Tetrahydrobiopterin availability in Parkinson's and Alzheimer's disease potential pathogenic mechanisms. Neurochem Res. 2007; 32:751–756. PMID: 17191137.
Article26. Takazawa C, Fujimoto K, Homma D, Sumi-Ichinose C, Nomura T, Ichinose H, Katoh S. A brain-specific decrease of the tyrosine hydroxylase protein in sepiapterin reductase-null mice--as a mouse model for Parkinson's disease. Biochem Biophys Res Commun. 2008; 367:787–792. PMID: 18201550.
Article27. Sundström E, Strömberg I, Tsutsumi T, Olson L, Jonsson G. Studies on the effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on central catecholamine neurons in C57BL/6 mice. Comparison with three other strains of mice. Brain Res. 1987; 405:26–38. PMID: 2882814.28. Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, Hirsch EC, Farrer M, Schapira AH, Halliday G. Missing pieces in the Parkinson's disease puzzle. Nat Med. 2010; 16:653–661. PMID: 20495568.
Article29. Nagatsu T, Sawada M. Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson's disease: possible implications of glial cells. J Neural Transm Suppl. 2006; (71):53–65. PMID: 17447416.
Article30. Francardo V, Recchia A, Popovic N, Andersson D, Nissbrandt H, Cenci MA. Impact of the lesion procedure on the profiles of motor impairment and molecular responsiveness to L-DOPA in the 6-hydroxydopamine mouse model of Parkinson's disease. Neurobiol Dis. 2011; 42:327–340. PMID: 21310234.
Article31. McMillan PJ, White SS, Franklin A, Greenup JL, Leverenz JB, Raskind MA, Szot P. Differential response of the central noradrenergic nervous system to the loss of locus coeruleus neurons in Parkinson's disease and Alzheimer's disease. Brain Res. 2011; 1373:240–252. PMID: 21147074.
Article32. Kitada T, Tomlinson JJ, Ao HS, Grimes DA, Schlossmacher MG. Considerations regarding the etiology and future treatment of autosomal recessive versus idiopathic Parkinson disease. Curr Treat Options Neurol. 2012; 14:230–240. PMID: 22547255.
Article33. Sedelis M, Hofele K, Auburger GW, Morgan S, Huston JP, Schwarting RK. MPTP susceptibility in the mouse: behavioral, neurochemical, and histological analysis of gender and strain differences. Behav Genet. 2000; 30:171–182. PMID: 11105391.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of a Silkworm Extract on Dopamine and Monoamine Oxidase-B Activity in an MPTP-induced Parkinsons Disease Model
- Changes of Locomotor Activity and Biogenic Amines by 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 Mice
- The electroretinographic change by the acute effects of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- Effects of (−)-Sesamin on Memory Deficits in MPTP-lesioned Mouse Model of Parkinson's Disease
- 1alpha,25-Dihydroxyvitamin D3 Protects Dopaminergic Neurons in Rodent Models of Parkinson's Disease through Inhibition of Microglial Activation