Korean J Physiol Pharmacol.
2013 Feb;17(1):73-79. 10.4196/kjpp.2013.17.1.73.
Anti-Inflammatory and Anti-Superbacterial Activity of Polyphenols Isolated from Black Raspberry
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea. kimwy@cau.ac.kr
- 2Department of Economics, Faculty of Business and Economics, Chung-Ang University, Seoul 156-756, Korea.
- KMID: 1432766
- DOI: http://doi.org/10.4196/kjpp.2013.17.1.73
Abstract
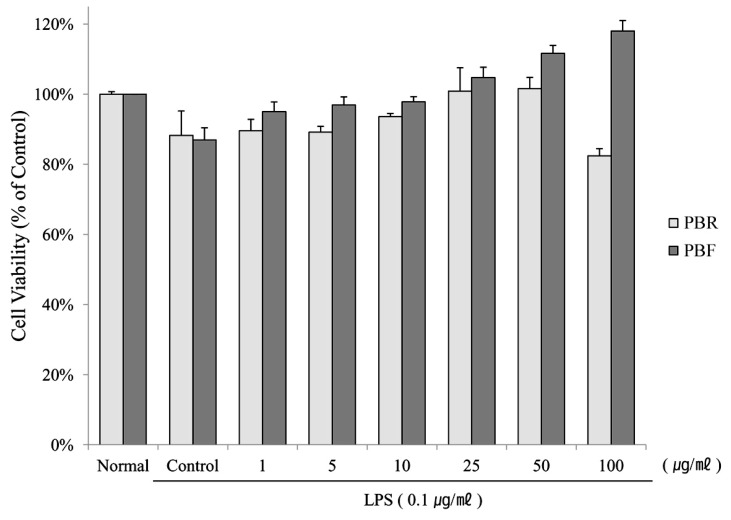
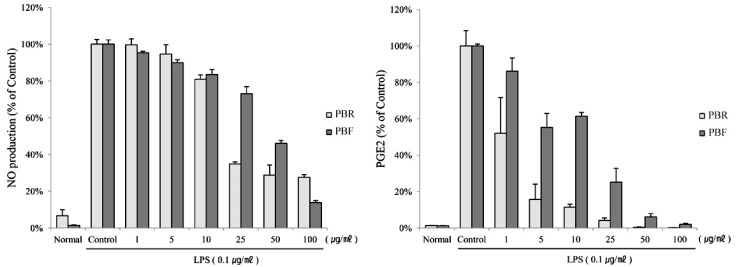
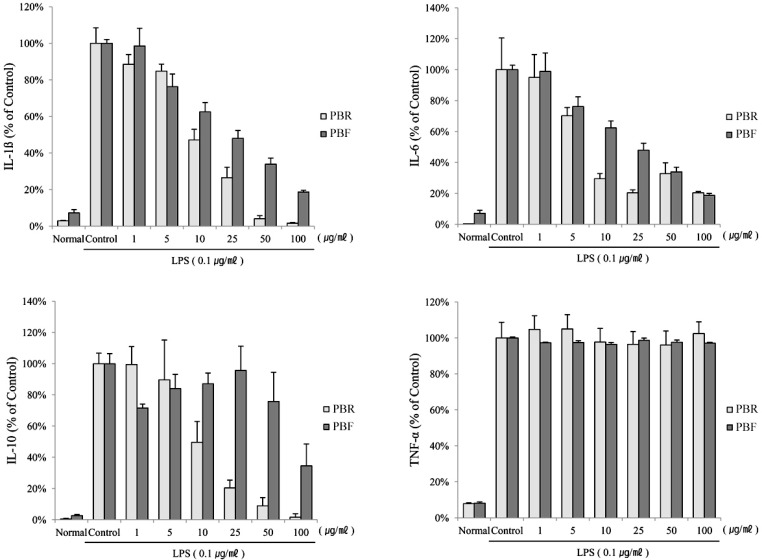
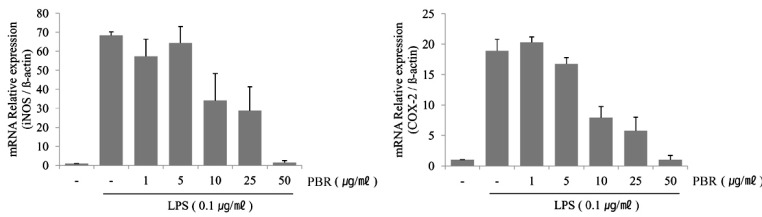
- The fruit of the black raspberry (Rubus coreanus Miquel) has been employed in traditional medicine, and recent studies have demonstrated its measureable biological activities. However, the root of the black raspberry has not been studied. Therefore, in this study, we evaluated the anti-inflammatory and antibacterial properties of the root and unripe fruit polyphenols of the black raspberry. Both polyphenols proved to have anti-inflammatory activity as evidenced by the decreased nitric oxide (NO), cytokines (IL-1beta , IL-6, and IL-10) and prostaglandin E2 (PGE2) levels in lipopolysaccharide (LPS)-stimulated RAW 264.7 murine macrophages. However, root polyphenols showed stronger anti-inflammatory activity than fruit polyphenols. LPS-induced mRNA and protein expressions of inducible NO synthase (iNOS) and cyclooxygenase (COX)-2 levels were also decreased, confirming the anti-inflammatory activity. Root polyphenols showed lethal activity against methicillin-resistant Staphylococcus aureus (MRSA), carbapenem-resistant Acinetobacter baumannii (CRAB), and Bacillus anthracis. In contrast, the black raspberry fruit did not demonstrate these properties. These data provide the first demonstration that black raspberry root has potential anti-inflammatory and anti-superbacterial properties that can be exploited as alternatives for use in the food and cosmetic industries and/or as pharmaceuticals.
Keyword
MeSH Terms
-
Acinetobacter baumannii
Bacillus anthracis
Cosmetics
Cytokines
Dinoprostone
Fruit
Interleukin-6
Macrophages
Medicine, Traditional
Methicillin-Resistant Staphylococcus aureus
Nitric Oxide
Nitric Oxide Synthase
Polyphenols
Prostaglandin-Endoperoxide Synthases
RNA, Messenger
Cosmetics
Cytokines
Dinoprostone
Interleukin-6
Nitric Oxide
Nitric Oxide Synthase
Polyphenols
Prostaglandin-Endoperoxide Synthases
RNA, Messenger
Figure
Reference
-
1. Kim EJ, Lee YJ, Shin HK, Park JH. Induction of apoptosis by the aqueous extract of Rubus coreanum in HT-29 human colon cancer cells. Nutrition. 2005; 21:1141–1148. PMID: 16308138.
Article2. Moon GS. Constituents and Uses of Medicinal Herbs. 1991. Seoul: Ilwolbooks.3. Heo J. Donguibogam. 1994. Seoul: Yeogang Publishing Co..4. Boscá L, Zeini M, Través PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005; 208:249–258. PMID: 15691589.
Article5. Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002; 2:787–795. PMID: 12360216.
Article6. Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003; 24:25–29. PMID: 12495721.
Article7. Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005; 23:787–819. PMID: 15771586.
Article8. Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995; 13:437–457. PMID: 7542010.
Article9. Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002; 23:144–150. PMID: 11864843.
Article10. Smith WL, Meade EA, DeWitt DL. Pharmacology of prostaglandin endoperoxide synthase isozymes-1 and -2. Ann N Y Acad Sci. 1994; 714:136–142. PMID: 8017762.11. Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001; 2:907–916. PMID: 11577346.
Article12. Laskin DL, Pendino KJ. Macrophages and inflammatory mediators in tissue injury. Annu Rev Pharmacol Toxicol. 1995; 35:655–677. PMID: 7598511.
Article13. Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998; 336:1–17. PMID: 9806879.
Article14. Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009; 48:1–12. PMID: 19035777.
Article15. Moellering RC Jr. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008; 46:1032–1037. PMID: 18444820.16. Esterly JS, Griffith M, Qi C, Malczynski M, Postelnick MJ, Scheetz MH. Impact of carbapenem resistance and receipt of active antimicrobial therapy on clinical outcomes of Acinetobacter baumannii bloodstream infections. Antimicrob Agents Chemother. 2011; 55:4844–4849. PMID: 21825287.
Article17. Routsi C, Pratikaki M, Platsouka E, Sotiropoulou C, Nanas S, Markaki V, Vrettou C, Paniara O, Giamarellou H, Roussos C. Carbapenem-resistant versus carbapenem-susceptible Acinetobacter baumannii bacteremia in a Greek intensive care unit: risk factors, clinical features and outcomes. Infection. 2010; 38:173–180. PMID: 20224962.
Article18. Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Hauer J, McDade J, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Tonat K. Anthrax as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 1999; 281:1735–1745. PMID: 10328075.19. Yang HM, Oh SM, Lim SS, Shin HK, Oh YS, Kim JK. Anti-inflammatory activities of Rubus coreanus depend on the degree of fruit ripening. Phytother Res. 2008; 22:102–107. PMID: 17724764.
Article20. Quave CL, Estévez-Carmona M, Compadre CM, Hobby G, Hendrickson H, Beenken KE, Smeltzer MS. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS One. 2012; 7:e28737. PMID: 22242149.
Article21. Pan MH, Lai CS, Wang YJ, Ho CT. Acacetin suppressed LPS-induced up-expression of iNOS and COX-2 in murine macrophages and TPA-induced tumor promotion in mice. Biochem Pharmacol. 2006; 72:1293–1303. PMID: 16949556.
Article22. Kim SJ. Curcumin suppresses the production of interleukin-6 in Prevotella intermedia lipopolysaccharide-activated RAW 264.7 cells. J Periodontal Implant Sci. 2011; 41:157–163. PMID: 21811692.23. Lee JW, Do JH. Determination of total phenolic compounds from the fruit and Rubus coreanum and antioxidative activity. J Korean Soc Food Sci Nutr. 2000; 29:943–947.24. Yoon I, Wee JH, Moon JH, Ahn TH, Park KH. Isolation and identification of quercetin with antioxidative activity from the fruits of Rubus coreanum Miquel. Korean J Food Sci Technol. 2003; 35:499–502.25. Jeon SK, Lee JW, Lee IS. Effect of antioxidant activity and induction of DNA damage on human gastric cancer cell by Rubus coreanus Miquel. J Life Sci. 2007; 17:1723–1728.
Article26. Cho YJ, Chun SS, Kwon HJ, Kim JH, Yoon SJ, Lee KH. Comparison of physiological activities between hot-water and ethanol extracts of Bokbunja (Rubus coreanum F.). J Korean Soc Food Sci Nutr. 2005; 34:790–796.27. Choi J, Lee KT, Ha J, Yun SY, Ko CD, Jung HJ, Park HJ. Antinociceptive and anti-inflammatory effects of Niga-ichigoside F1 and 23-hydroxytormentic acid obtained from Rubus coreanus. Biol Pharm Bull. 2003; 26:1436–1441. PMID: 14519951.
Article28. Nordmann P, Poirel L. Emerging carbapenemases in Gram-negative aerobes. Clin Microbiol Infect. 2002; 8:321–331. PMID: 12084099.
Article29. Beck-Sagué CM, Jarvis WR, Brook JH, Culver DH, Potts A, Gay E, Shotts BW, Hill B, Anderson RL, Weinstein MP. Epidemic bacteremia due to Acinetobacter baumannii in five intensive care units. Am J Epidemiol. 1990; 132:723–733. PMID: 2403113.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-Inflammatory and Anti-Superbacterial Properties of Sulforaphane from Shepherd's Purse
- Black Raspberry Improved Lipid Profiles and Vascular Endothelial Function in Patients with Metabolic Syndrome: A Subgroup Analysis of Statin Naïve Participants
- The Effect of Polyphenols Isolated from Cynanchi wilfordii Radix with Anti-inflammatory, Antioxidant, and Anti-bacterial Activity
- Molecular Targets of Dietary Polyphenols with Anti-inflammatory Properties
- Anti-microbial and Anti-inflammatory Activity of New 4-methoxy-3-(methoxymethyl) Phenol and (E)-N′-(5-bromo-2-methoxybenzylidene)-4-methoxy Benzohydrazide Isolated from Calotropis gigantean white