Korean J Physiol Pharmacol.
2013 Feb;17(1):31-36. 10.4196/kjpp.2013.17.1.31.
The Inhibitory Effect of Eupatilin on the Agonist-Induced Regulation of Vascular Contractility
- Affiliations
-
- 1Department of Pharmacology, College of Pharmacy, Catholic University of Daegu, Gyeongbuk 712-702, Korea.
- 2Department of Physical Therapy, College of Health Science, Korea University, Seoul 136-701, Korea.
- 3Department of Pharmacology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea. jhjeong3@cau.ac.kr
- 4Research Institute for Translational System Biomics, Chung-Ang University, Seoul 156-756, Korea.
- KMID: 1432760
- DOI: http://doi.org/10.4196/kjpp.2013.17.1.31
Abstract
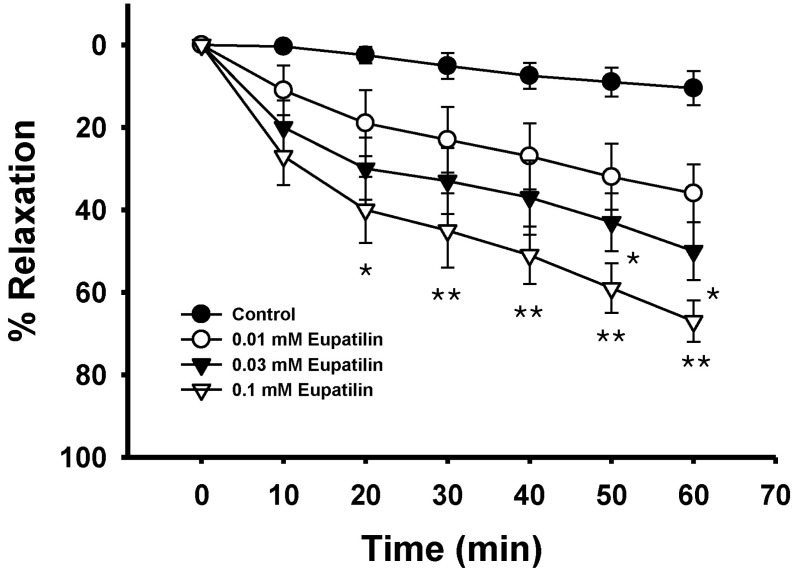
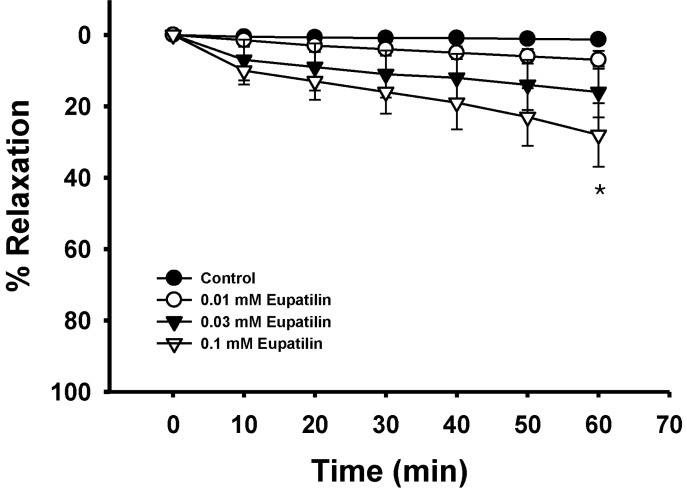
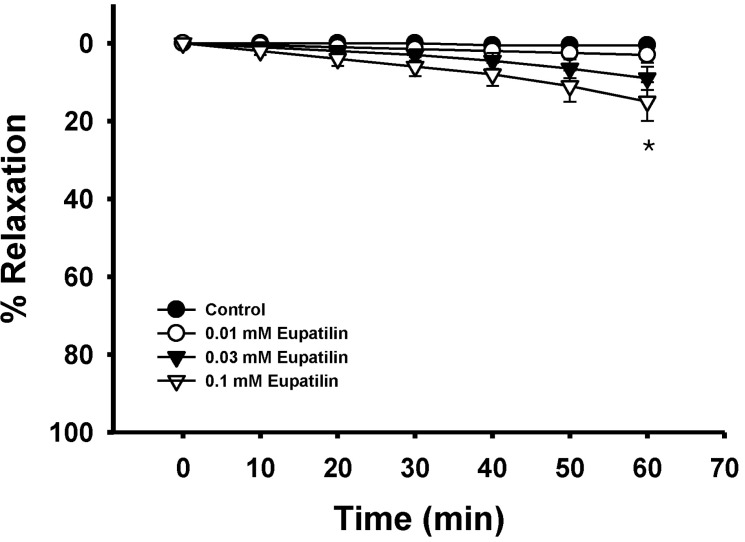
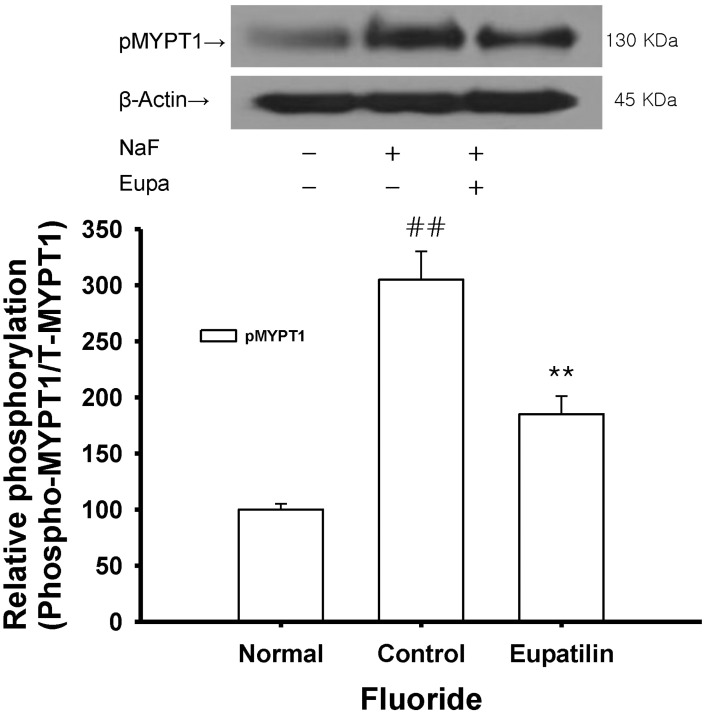
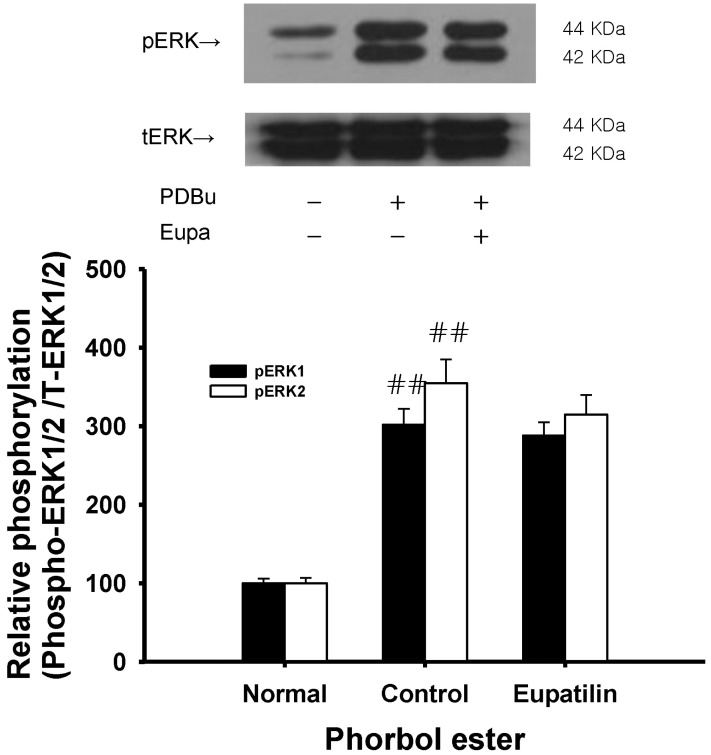
- The present study was undertaken to investigate the influence of eupatilin on vascular smooth muscle contractility and to determine the mechanism involved. Denuded aortic rings from male rats were used and isometric contractions were recorded and combined with molecular experiments. Eupatilin more significantly relaxed fluoride-induced vascular contraction than thromboxane A2 or phorbol ester-induced contraction suggesting as a possible anti-hypertensive on the agonist-induced vascular contraction regardless of endothelial nitric oxide synthesis. Furthermore, eupatilin significantly inhibited fluoride-induced increases in pMYPT1 levels. On the other hand, it didn't significantly inhibit phorbol ester-induced increases in pERK1/2 levels suggesting the mechanism involving the primarily inhibition of Rho-kinase activity and the subsequent phosphorylation of MYPT1. This study provides evidence regarding the mechanism underlying the relaxation effect of eupatilin on agonist-induced vascular contraction regardless of endothelial function.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Trichostatin A Modulates Angiotensin II-induced Vasoconstriction and Blood Pressure Via Inhibition of p66shc Activation
Gun Kang, Yu Ran Lee, Hee Kyoung Joo, Myoung Soo Park, Cuk-Seong Kim, Sunga Choi, Byeong Hwa Jeon
Korean J Physiol Pharmacol. 2015;19(5):467-472. doi: 10.4196/kjpp.2015.19.5.467.
Reference
-
1. Kalemba D, Kusewicz D, Swiader K. Antimicrobial properties of the essential oil of Artemisia asiatica Nakai. Phytother Res. 2002; 16:288–291. PMID: 12164281.
Article2. Song HJ, Shin CY, Oh TY, Sohn UD. The protective effect ofeupatilin on indomethacin-induced cell damage in cultured feline ileal smooth muscle cells: involvement of HO-1 and ERK. J Ethnopharmacol. 2008; 118:94–101. PMID: 18440740.3. Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994; 372:231–236. PMID: 7969467.
Article4. Somlyo AP, Somlyo AV. From pharmacomechanical coupling to G-proteins and myosin phosphatase. Acta Physiol Scand. 1998; 164:437–448. PMID: 9887967.
Article5. Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997; 389:990–994. PMID: 9353125.
Article6. Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-dependent activation of Rho and Rho kinasein membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res. 2003; 93:548–556. PMID: 12919947.7. Kitazawa T, Masuo M, Somlyo AP. G protein-mediated inhibition of myosin light-chain phosphatase invascular smooth muscle. Proc Natl Acad Sci USA. 1991; 88:9307–9310. PMID: 1656467.8. Wier WG, Morgan KG. Alpha1-adrenergic signaling mechanisms in contraction of resistance arteries. Rev Physiol Biochem Pharmacol. 2003; 150:91–139. PMID: 12884052.9. Kanaho Y, Moss J, Vaughan M. Mechanism of inhibition of transducin GTPase activity by fluoride and aluminum. J Biol Chem. 1985; 260:11493–11497. PMID: 2995338.
Article10. Blackmore PF, Exton JH. Studies on the hepatic calciummobilizing activity of aluminum fluoride and glucagon. Modulation by cAMP and phorbol myristate acetate. J Biol Chem. 1986; 261:11056–11063. PMID: 2426266.
Article11. Cockcroft S, Taylor JA. Fluoroaluminates mimic guanosine 5'-[gamma-thio]triphosphate in activating the polyphosphoinositide phosphodiesterase of hepatocyte membranes. Role for the guanine nucleotide regulatory protein Gp in signal transduction. Biochem J. 1987; 241:409–414. PMID: 3036062.12. Jeon SB, Jin F, Kim JI, Kim SH, Suk K, Chae SC, Jun JE, Park WH, Kim IK. A role for Rho kinase in vascular contraction evoked by sodium fluoride. Biochem Biophys Res Commun. 2006; 343:27–33. PMID: 16527249.
Article13. Wilson DP, Susnjar M, Kiss E, Sutherland C, Walsh MP. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem J. 2005; 389:763–774. PMID: 15823093.14. Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem. 2004; 279:34496–34504. PMID: 15194681.
Article15. Zeng YY, Benishin CG, Pang PK. Guanine nucleotide binding proteins may modulate gating of calcium channels in vascular smooth muscle. I. Studies with fluoride. J Pharmacol Exp Ther. 1989; 250:343–351. PMID: 2473190.16. Chabre M. Aluminofluoride and beryllofluoride complexes: a new phosphate analogs in enzymology. Trends Biochem Sci. 1990; 15:6–10. PMID: 2180149.17. Bigay J, Deterre P, Pfister C, Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985; 191:181–185. PMID: 3863758.18. Shenolikar S, Nairn AC. Protein phosphatases: recent progress. Adv Second Messenger Phosphoprotein Res. 1991; 23:1–121. PMID: 1847640.19. Tsai MH, Jiang MJ. Rho-kinase-mediated regulation of receptor-agonist-stimulated smooth muscle contraction. Pflugers Arch. 2006; 453:223–232. PMID: 16953424.
Article20. Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000; 522:177–185. PMID: 10639096.
Article21. Pfitzer G. Invited review: regulation of myosin phosphorylation in smooth muscle. J Appl Physiol. 2001; 91:497–503. PMID: 11408468.22. Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem. 1996; 271:20246–20249. PMID: 8702756.
Article23. Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Gui P, Hill MA, Wilson E. Regulation of ion channels by protein tyrosine phosphorylation. Am J Physiol Heart Circ Physiol. 2001; 281:H1835–H1862. PMID: 11668044.
Article24. Low AM. Role of tyrosine kinase on Ca2+ entry and refilling of agonist-sensitive Ca2+ stores in vascular smooth muscles. Can J Physiol Pharmacol. 1996; 74:298–304. PMID: 8773410.25. Deng JT, Van Lierop JE, Sutherland C, Walsh MP. Ca2+-independent smooth muscle contraction. a novel function for integrin-linked kinase. J Biol Chem. 2001; 276:16365–16373. PMID: 11278951.26. Murányi A, MacDonald JA, Deng JT, Wilson DP, Haystead TA, Walsh MP, Erdodi F, Kiss E, Wu Y, Hartshorne DJ. Phosphorylation of the myosin phosphatase target subunit by integrin-linked kinase. Biochem J. 2002; 366:211–216. PMID: 12030846.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Inhibitory Effect of Shikonin on the Agonist-Induced Regulation of Vascular Contractility
- The Inhibitory Effect of Apigenin on the Agonist-Induced Regulation of Vascular Contractility via Calcium Desensitization-Related Pathways
- The inhibitory effect of eupatilin on Helicobacter pylori-induced release of leukotriene D4 in the human neutrophils and gastric mucosal cells
- Endothelium-Independent Effect of Fisetin on the Agonist-Induced Regulation of Vascular Contractility
- Cardamonin inhibits agonist-induced vascular contractility via Rho-kinase and MEK inhibition