Immune Netw.
2013 Apr;13(2):55-62. 10.4110/in.2013.13.2.55.
Swiprosin-1 Expression Is Up-Regulated through Protein Kinase C-theta and NF-kappaB Pathway in T Cells
- Affiliations
-
- 1School of Life Sciences, Immune Synapse Research Center and Cell Dynamics Research Center, Gwangju Institute of Science and Technology, Gwangju 500-712, Korea. cdjun@gist.ac.kr
- KMID: 1431926
- DOI: http://doi.org/10.4110/in.2013.13.2.55
Abstract
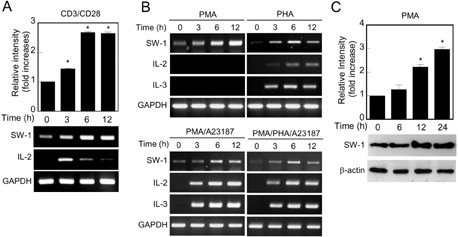
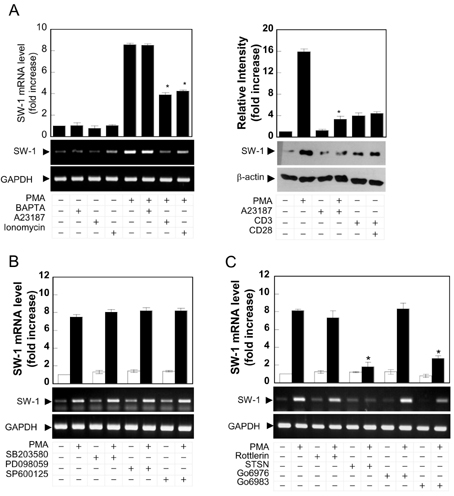
- Swiprosin-1 exhibits the highest expression in CD8+ T cells and immature B cells and has been proposed to play a role in lymphocyte biology through actin remodeling. However, regulation of swiprosin-1 gene expression is poorly understood. Here we report that swiprosin-1 is up-regulated in T cells by PKC pathway. Targeted inhibition of the specific protein kinase C (PKC) isotypes by siRNA revealed that PKC-theta is involved in the expression of swiprosin-1 in the human T cells. In contrast, down-regulation of swiprosin-1 by A23187 or ionomycin suggests that calcium-signaling plays a negative role. Interestingly, swiprosin-1 expression is only reduced by treatment with NF-kappaB inhibitors but not by NF-AT inhibitor, suggesting that the NF-kappaB pathway is critical for regulation of swiprosin-1 expression. Collectively, these results suggest that swiprosin-1 is a PKC-theta-inducible gene and that it may modulate the late phase of T cell activation after antigen challenge.
Keyword
MeSH Terms
Figure
Reference
-
1. Vuadens F, Rufer N, Kress A, Corthésy P, Schneider P, Tissot JD. Identification of swiprosin 1 in human lymphocytes. Proteomics. 2004. 4:2216–2220.
Article2. Avramidou A, Kroczek C, Lang C, Schuh W, Jäck HM, Mielenz D. The novel adaptor protein Swiprosin-1 enhances BCR signals and contributes to BCR-induced apoptosis. Cell Death Differ. 2007. 14:1936–1947.
Article3. Ramesh TP, Kim YD, Kwon MS, Jun CD, Kim SW. Swiprosin-1 regulates cytokine expression of human mast cell line HMC-1 through actin remodeling. Immune Netw. 2009. 9:274–284.
Article4. Blagoev B, Ong SE, Kratchmarova I, Mann M. Temporal analysis of phosphotyrosine-dependent signaling networks by quantitative proteomics. Nat Biotechnol. 2004. 22:1139–1145.
Article5. Mielenz D, Vettermann C, Hampel M, Lang C, Avramidou A, Karas M, Jäck HM. Lipid rafts associate with intracellular B cell receptors and exhibit a B cell stage-specific protein composition. J Immunol. 2005. 174:3508–3517.
Article6. Piragyte I, Jun CD. Actin engine in immunological synapse. Immune Netw. 2012. 12:71–83.
Article7. Meng X, Wilkins JA. Compositional characterization of the cytoskeleton of NK-like cells. J Proteome Res. 2005. 4:2081–2087.
Article8. Thylur RP, Kim YD, Kwon MS, Oh HM, Kwon HK, Kim SH, Im SH, Chun JS, Park ZY, Jun CD. Swiprosin-1 is expressed in mast cells and up-regulated through the protein kinase C beta I/eta pathway. J Cell Biochem. 2009. 108:705–715.
Article9. Hayashi K, Altman A. Protein kinase C theta (PKCtheta): a key player in T cell life and death. Pharmacol Res. 2007. 55:537–544.10. Freeley M, Long A. Regulating the regulator: Phosphorylation of PKC θ in T Cells. Front Immunol. 2012. 3:227.
Article11. Kwon MJ, Wang R, Ma J, Sun Z. PKC-θ is a drug target for prevention of T cell-mediated autoimmunity and allograft rejection. Endocr Metab Immune Disord Drug Targets. 2010. 10:367–372.
Article12. Manicassamy S, Yin D, Zhang Z, Molinero LL, Alegre ML, Sun Z. A critical role for protein kinase C-theta-mediated T cell survival in cardiac allograft rejection. J Immunol. 2008. 181:513–520.
Article13. Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002. 2:933–944.
Article14. Shahabi NA, McAllen K, Sharp BM. Stromal cell-derived factor 1-alpha (SDF)-induced human T cell chemotaxis becomes phosphoinositide 3-kinase (PI3K)-independent: role of PKC-theta. J Leukoc Biol. 2008. 83:663–671.
Article15. Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem J. 2003. 376:545–552.
Article16. Michalczyk I, Sikorski AF, Kotula L, Junghans RP, Dubielecka PM. The emerging role of protein kinase Cθ in cytoskeletal signaling. J Leukoc Biol. 2013. 93:319–327.
Article17. Manicassamy S, Gupta S, Huang Z, Sun Z. Protein kinase C-theta-mediated signals enhance CD4XMLLink_XYZ T cell survival by up-regulating Bcl-xL. J Immunol. 2006. 176:6709–6716.
Article18. Isakov N, Altman A. Protein kinase C(theta) in T cell activation. Annu Rev Immunol. 2002. 20:761–794.19. Altman A, Villalba M. Protein kinase C-theta (PKC theta): a key enzyme in T cell life and death. J Biochem. 2002. 132:841–846.20. Boschelli DH. Small molecule inhibitors of PKCTheta as potential antiinflammatory therapeutics. Curr Top Med Chem. 2009. 9:640–654.
Article21. Lanzavecchia A. Understanding the mechanisms of sustained signaling and T cell activation. J Exp Med. 1997. 185:1717–1719.
Article22. Gardner P. Calcium and T lymphocyte activation. Cell. 1989. 59:15–20.
Article23. Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997. 15:707–747.24. Chan AC, Desai DM, Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immunol. 1994. 12:555–592.
Article25. Bootman MD, Berridge MJ. The elemental principles of calcium signaling. Cell. 1995. 83:675–678.
Article26. Vega IE, Traverso EE, Ferrer-Acosta Y, Matos E, Colon M, Gonzalez J, Dickson D, Hutton M, Lewis J, Yen SH. A novel calcium-binding protein is associated with tau proteins in tauopathy. J Neurochem. 2008. 106:96–106.
Article27. Dütting S, Brachs S, Mielenz D. Fraternal twins: Swiprosin-1/EFhd2 and Swiprosin-2/EFhd1, two homologous EF-hand containing calcium binding adaptor proteins with distinct functions. Cell Commun Signal. 2011. 9:2.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Swiprosin-1 Regulates Cytokine Expression of Human Mast Cell Line HMC-1 through Actin Remodeling
- PMA-induced up-regulation of MMP-9 is regulated by a PKCalpha-NF-kappaB cascade in human lung epithelial cells
- Role of protein kinases on NF- kappaB activation and cell death in bovine cerebral endothelial cells
- ASK1 is Involved in EBV LMP1-induced NF-kappaB Activation
- NF-kappaB Activation in T Helper 17 Cell Differentiation