Lab Anim Res.
2013 Jun;29(2):84-95. 10.5625/lar.2013.29.2.84.
Red Liriope platyphylla stimulated the insulin secretion through the regulation of calcium concentration in rat insulinoma cells and animal models
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2College of Pharmacy, Pusan National University, Busan, Korea.
- KMID: 1431339
- DOI: http://doi.org/10.5625/lar.2013.29.2.84
Abstract
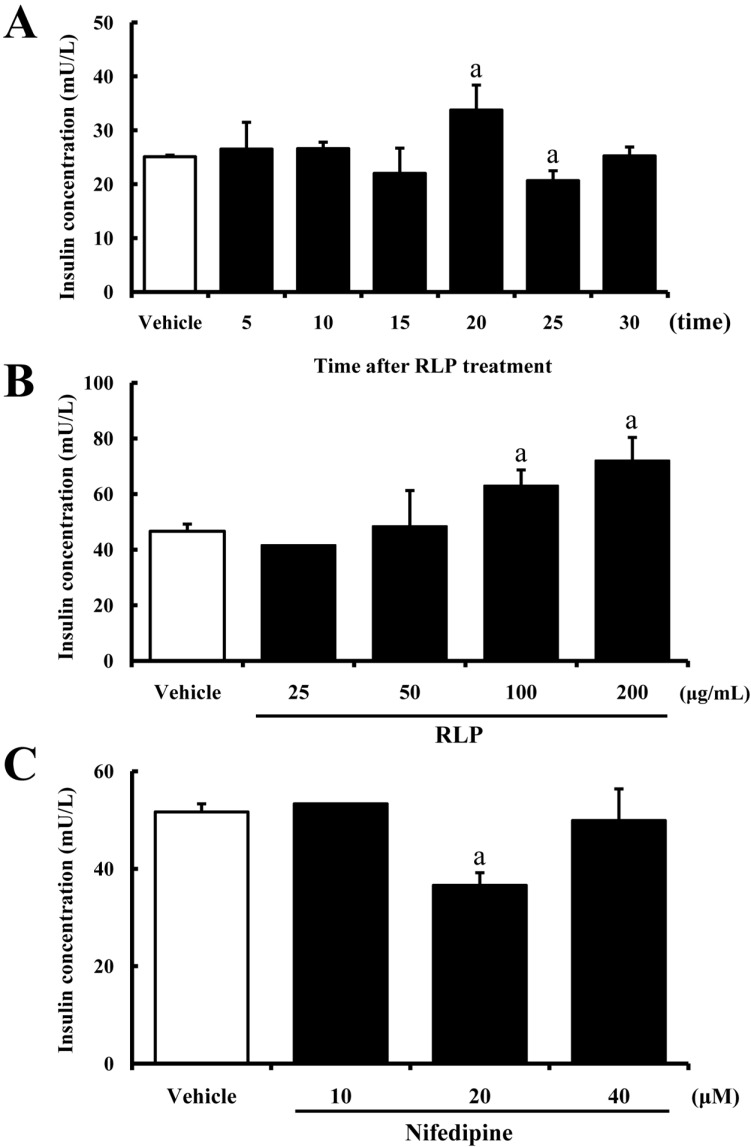
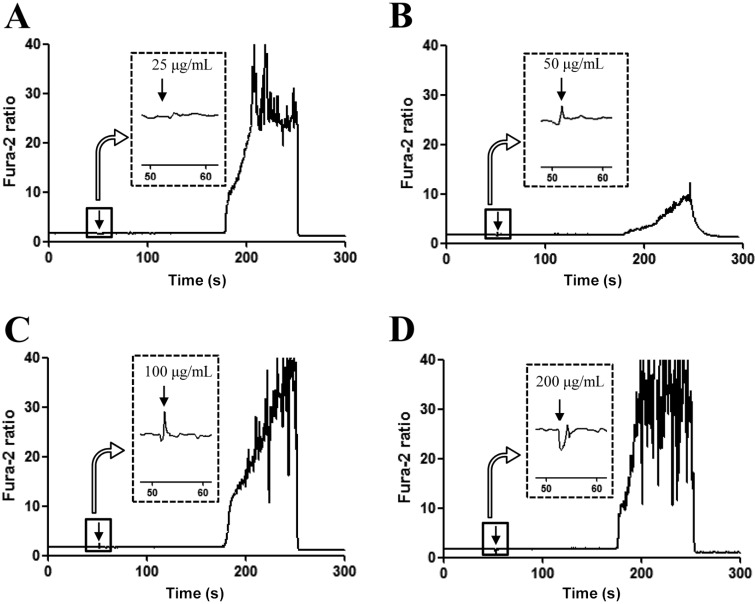
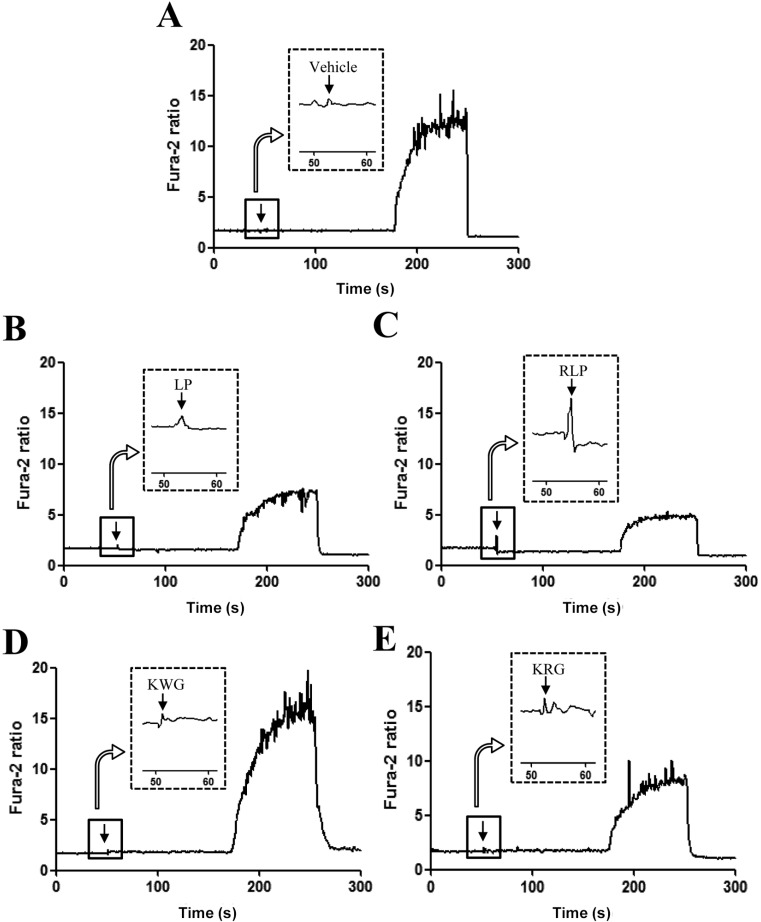
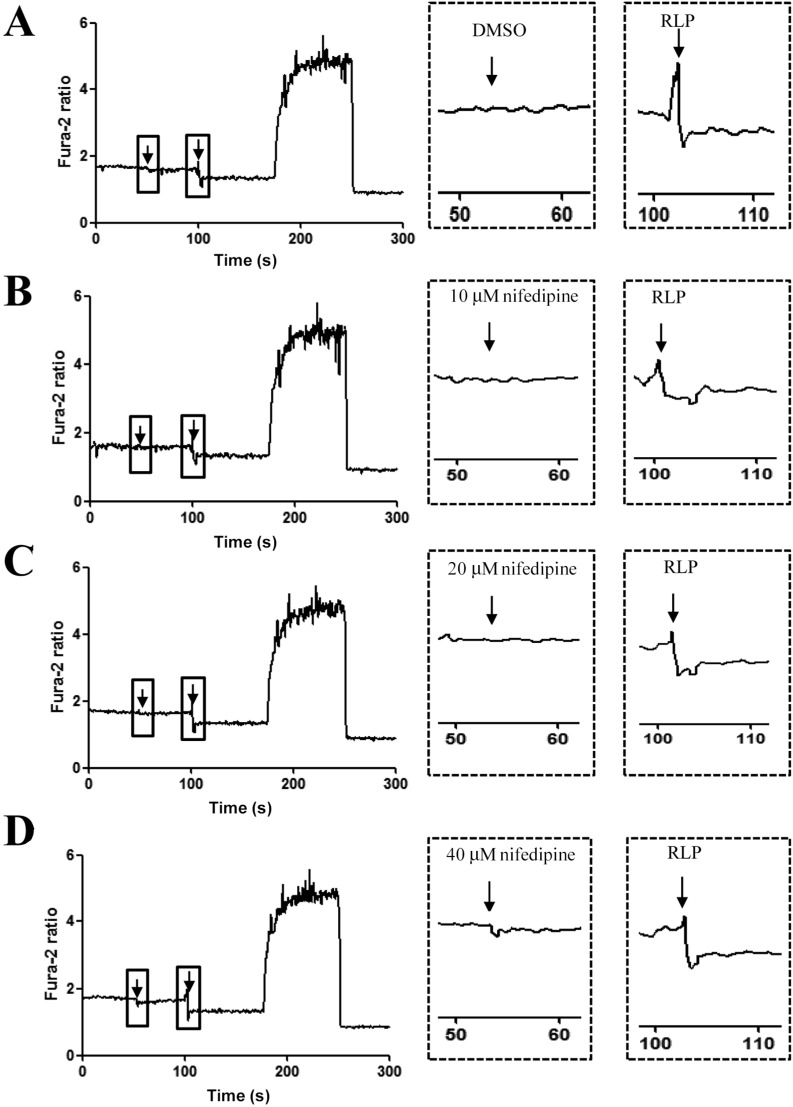
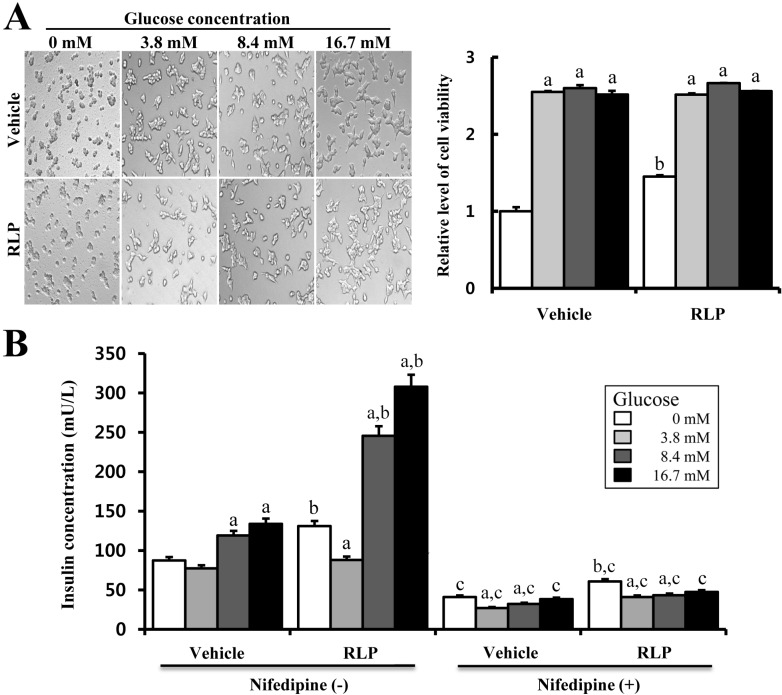
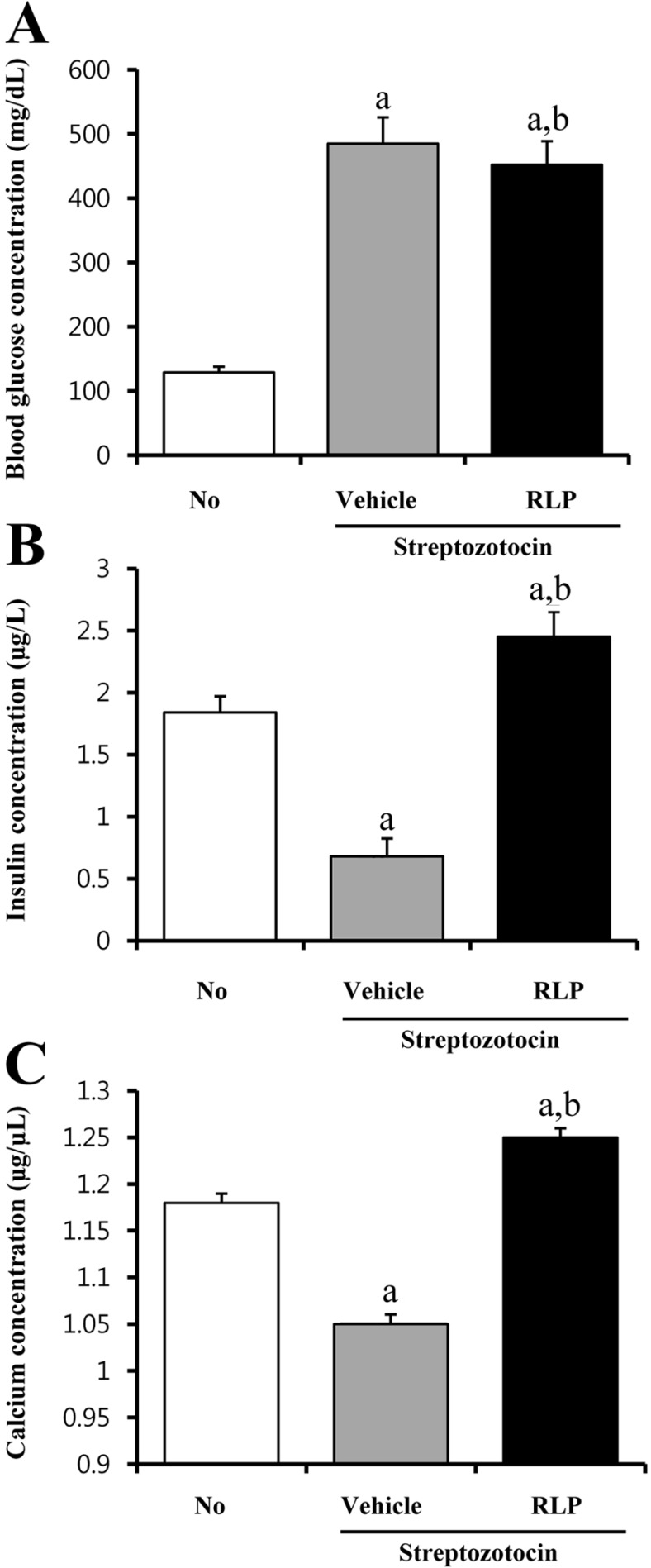
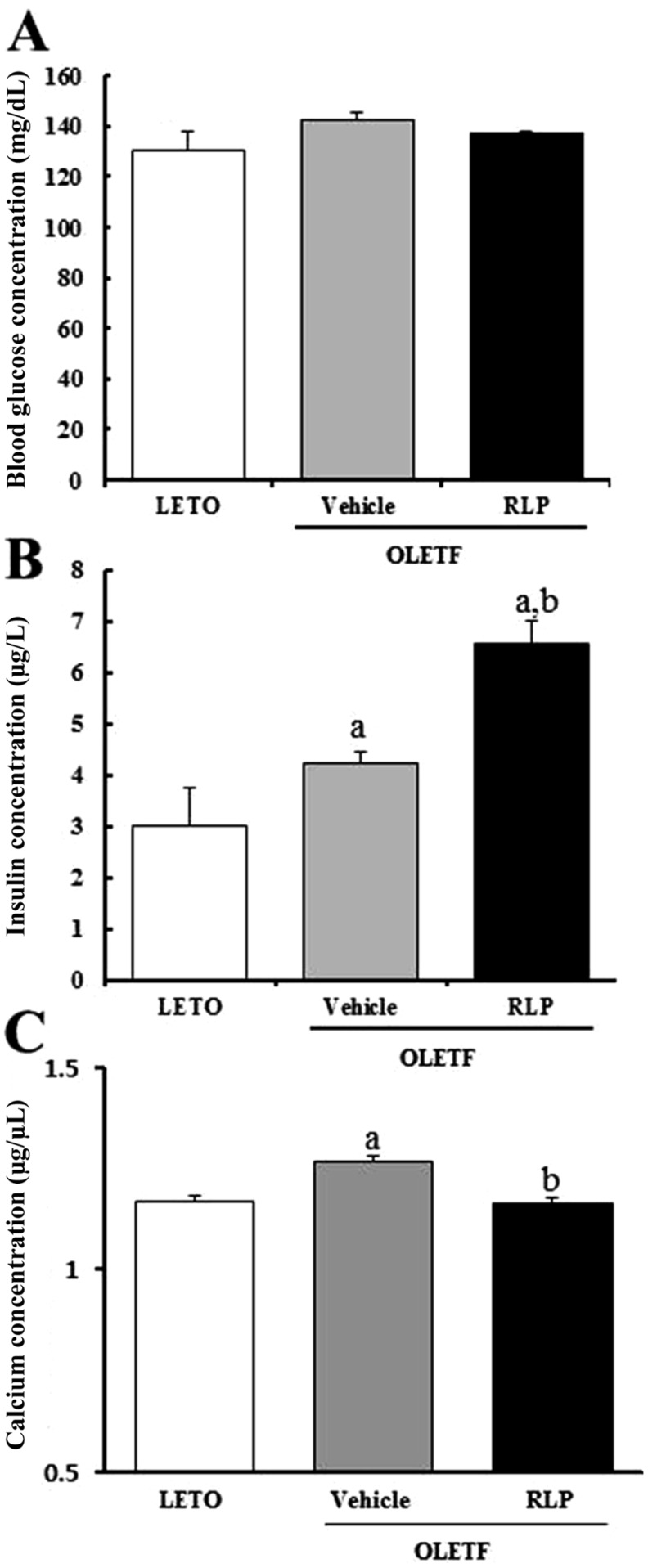
- The aim of this study was to investigate the effects of Red L. platyphylla (RLP) on calcium and glucose levels during insulin secretion. To achieve this, alteration of insulin and calcium concentrations was measured in rat insulinoma-1 (INS-1) cells and animal models in response to RLP treatment. In INS-1 cells, maximum secretion of insulin was detected upon treatment with 200 microg/mL of RLP for 20 min. Nifedipine, an L-type calcium channel blocker, effectively inhibited insulin secretion from INS-1 cells. Regarding calcium levels, the maximum concentration of intracellular calcium in INS-1 cells was obtained by treatment with 100 microg/mL of RLP, whereas this level was reduced under conditions of 200 microg/mL of RLP. Further, RLP-treated INS-1 cells showed a higher level of intracellular calcium than that of L. platyphylla (LP), Korea White Ginseng (KWG), or Korea Red Ginseng (KRG)-treated cells. This RLP-induced increase in intracellular calcium was abrogated but not completely abolished upon treatment with 40 microM nifedipine in a dose-dependent manner. Furthermore, the insulin level was dramatically elevated upon co-treatment with high concentrations of glucose and RLP, whereas it was maintained at a low level in response to glucose and RLP co-treatment at low concentrations. In an animal experiment, the serum concentration of calcium increased or decreased upon RLP treatment according to glucose level compared to vehicle treatment. Therefore, these results suggest that insulin secretion induced by RLP treatment may be tightly correlated with calcium regulation, which suggests RLP is an excellent candidate for diabetes treatment.
Keyword
MeSH Terms
Figure
Reference
-
1. Huh MK, Huh HW, Choi JS, Lee BK. Genetic diversity and population structure of Liriope platyphylla (Liliaceae) in Korea. J Life Sci. 2007; 17:328–333.2. Lee YC, Lee JC, Seo YB, Kook YB. Liriopistuber inhibit OVA-induced airway inflammation and bronchial hyperresponsiveness in murine model of asthma. J Ethnopharmacol. 2005; 101(1-3):144–152. PMID: 15982838.3. Choi SB, Wha JD, Park S. The insulin sensitizing effect of homoisoflavone-enriched fraction in Liriope platyphylla Wang et Tang via PI3-kinase pathway. Life Sci. 2004; 75(22):2653–2664. PMID: 15369701.4. Hur J, Lee P, Kim J, Kim AJ, Kim H, Kim SY. Induction of nerve growth factor by butanol fraction of Liriope platyphylla in C6 and primary astrocyte cells. Biol Pharm Bull. 2004; 27(8):1257–1260. PMID: 15305032.5. Jeong S, Chae K, Jung YS, Rho YH, Lee J, Ha J, Yoon KH, Kim GC, Oh KS, Shin SS, Yoon M. The Korean traditional medicine Gyeongshingangjeehwan inhibits obesity through the regulation of leptin and PPARalpha action in OLETF rats. J Ethnopharmacol. 2008; 119(2):245–251. PMID: 18674606.6. Hur J, Lee P, Moon E, Kang I, Kim SH, Oh MS, Kim SY. Neurite outgrowth induced by spicatoside A, a steroidal saponin, via the tyrosine kinase A receptor pathway. Eur J Pharmacol. 2009; 620(1-3):9–15. PMID: 19695245.
Article7. Kim JE, Lee YK, Nam SH, Choi SI, Goo JS, Jang MJ, Lee HS, Son HJ, Lee CY, Hwang DY. The symptoms of atopic dermatitis in NC/Nga mice were significantly relieved by the water extract of Liriope platyphylla. Lab Anim Res. 2010; 26:377–384.8. Lee YK, Kim JE, Nam SH, Goo JS, Choi SI, Choi YH, Bae CJ, Woo JM, Cho JS, Hwang DY. Differential regulation of the biosynthesis of glucose transporters by the PI3-K and MAPK pathways of insulin signaling by treatment with novel compounds from Liriope platyphylla. Int J Mol Med. 2011; 27(3):319–327. PMID: 21165549.
Article9. Kim JE, Nam SH, Choi SI, Hwang IS, Lee HR, Jang MJ, Lee CY, Soon HJ, Lee HS, Kim HS, Kang BC, Hong JT, Hwang DY. Aqueous extracts of Liriope platyphylla are tightly-regulated by insulin secretion from pancreatic islets and by increased glucose uptake through glucose transporters expressed in liver hepatocytes. Biomol Ther. 2011; 19(3):348–356.10. Kim K, Kim HY. Korean red ginseng stimulates insulin release from isolated rat pancreatic islets. J Ethnopharmacol. 2008; 120(2):190–195. PMID: 18773949.
Article11. Choi SI, Lee HR, Goo JS, Kim JE, Nam SH, Hwang IS, Lee YJ, Prak SH, Lee HS, Lee JS, Jang IS, Son HJ, Hwang DY. Effects of steaming time and frequency for manufactured red Liriope platyphylla on the insulin secretion ability and insulin receptor signaling pathway. Lab Anim Res. 2011; 27(2):117–126. PMID: 21826171.12. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985; 260(6):3440–3450. PMID: 3838314.13. Bouche C, Lopez X, Fleischman A, Cypess AM, O'Shea S, Stefanovski D, Bergman RN, Rogatsky E, Stein DT, Kahn CR, Kulkarni RN, Goldfine AB. Insulin enhances glucose-stimulated insulin secretion in healthy humans. Proc Natl Acad Sci USA. 2010; 107(10):4770–4775. PMID: 20176932.
Article14. van Geijn HP, Lenglet JE, Bolte AC. Nifedipine trials: effectiveness and safety aspects. BJOG. 2005; 112:79–83. PMID: 15715601.15. Chakravarthy MV, Semenkovich CF. The ABCs of beta-cell dysfunction in type 2 diabetes. Nat Med. 2007; 13(3):241–242. PMID: 17342111.16. MacDonald MJ, Fahien LA, Brown LJ, Hasan NM, Buss JD, Kendrick MA. Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am J Physiol Endocrinol Metab. 2005; 288(1):E1–E15. PMID: 15585595.
Article17. Graves TK, Hinkle PM. Ca(2+)-induced Ca(2+) release in the pancreatic beta-cell: direct evidence of endoplasmic reticulum Ca(2+) release. Endocrinology. 2003; 144(8):3565–3574. PMID: 12865339.18. Lang J. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur J Biochem. 1999; 259(1-2):3–17. PMID: 9914469.
Article19. Kasabri V, Flatt PR, Abdel-Wahab YH. Terminaliabellirica stimulates the secretion and action of insulin and inhibits starch digestion and protein glycation in vitro. Br J Nutr. 2010; 103(2):212–217. PMID: 19723351.20. Al-Romaiyan A, Jayasri MA, Mathew TL, Huang GC, Amiel S, Jones PM, Persaud SJ. Costuspictus extracts stimulate insulin secretion from mouse and human islets of Langerhans in vitro. Cell Physiol Biochem. 2010; 26(6):1051–1058. PMID: 21220936.21. Liu B, Asare-Anane H, Al-Romaiyan A, Huang G, Amiel SA, Jones PM, Persaud SJ. Characterisation of the insulinotropic activity of an aqueous extract of Gymnema sylvestre in mouse beta-cells and human islets of Langerhans. Cell Physiol Biochem. 2009; 23(1-3):125–132. PMID: 19255507.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Steaming Time and Frequency for Manufactured Red Liriope platyphylla on the Insulin Secretion Ability and Insulin Receptor Signaling Pathway
- Precautionary effects of Red Liriope platyphylla on NGF secretion and Abeta42 deposition under the preclinical stage of Alzheimer's disease in Tg2576 mice
- Aqueous extract of Liriope platyphylla, a traditional Chinese medicine, significantly inhibits abdominal fat accumulation and improves glucose regulation in OLETF type II diabetes model rats
- Resistin Inhibits Insulin Secretion Through Inhibition of Insulin Granule Docking Via Downregulation of Rab3A in Pancreatic Beta-cells
- Ca Effects on Synthesis and Secretion of Insulin-like Growth Factor(IGF-I) and IGF-Binding Proteins by the Perfased Rat Liver