Korean J Physiol Pharmacol.
2013 Apr;17(2):121-125. 10.4196/kjpp.2013.17.2.121.
Ginsenosides Have a Suppressive Effect on c-Fos Expression in Brain and Reduce Cardiovascular Responses Increased by Noxious Stimulation to the Rat Tooth
- Affiliations
-
- 1Dental Science Research Institute, Medical Research Center for Biomineralization Disorders, School of Dentistry, Chonnam National University, Gwangju 500-757, Korea. wjkim@jnu.ac.kr
- 2Department of Oral Physiology, School of Dentistry, Chonnam National University, Gwangju 500-757, Korea.
- 3Department of Orthodontics, School of Dentistry, Chonnam National University, Gwangju 500-757, Korea.
- 4Department of Oral Anatomy, School of Dentistry, Chonnam National University, Gwangju 500-757, Korea.
- KMID: 1429382
- DOI: http://doi.org/10.4196/kjpp.2013.17.2.121
Abstract
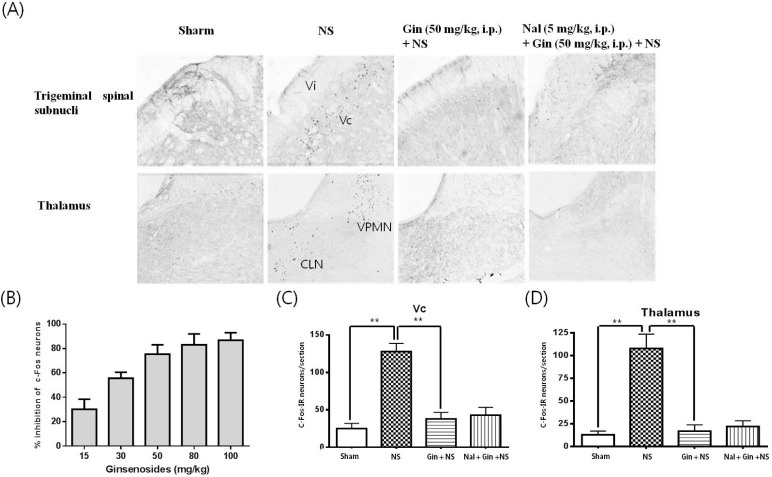
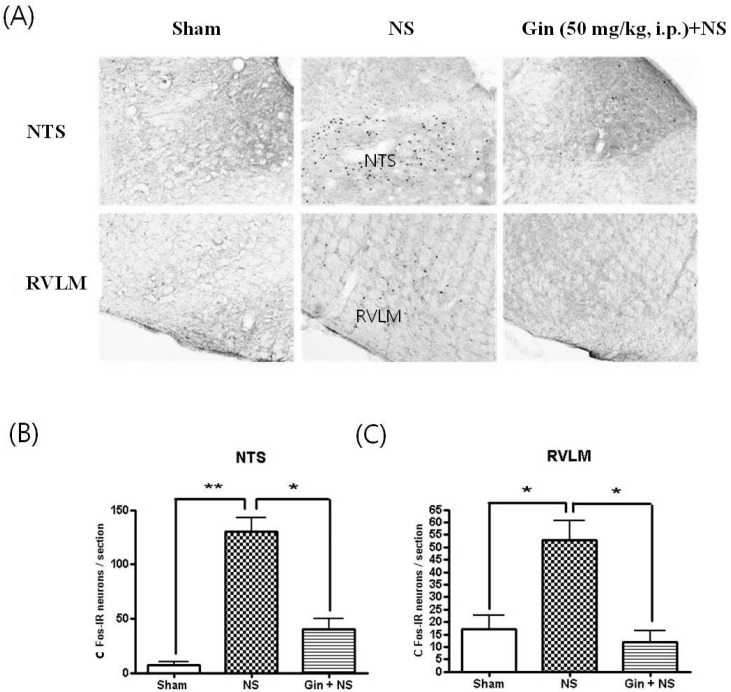
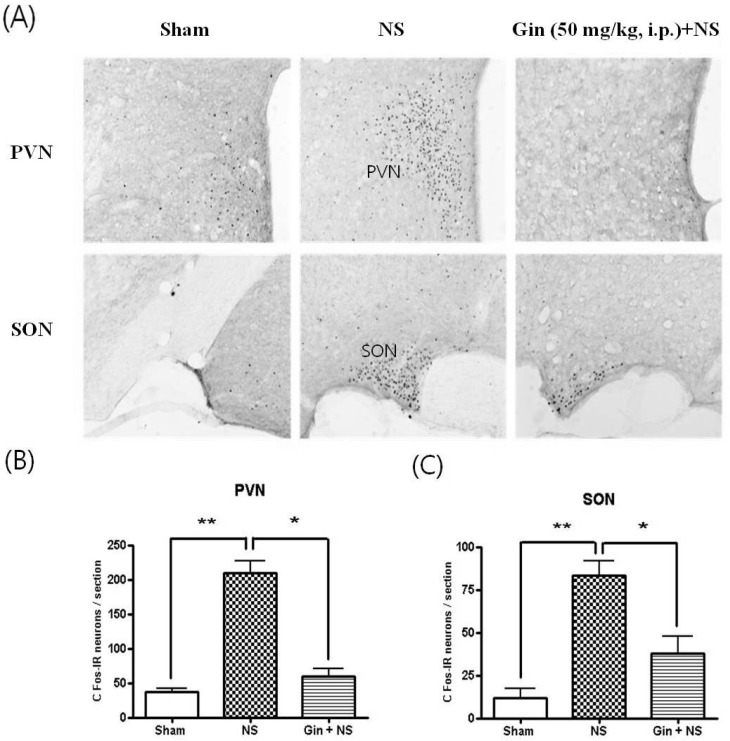
- The purpose of this study is to investigate the antinociceptive effects of ginsenosides on toothache. c-Fos immunoreactive (IR) neurons were examined after noxious intrapulpal stimulation (NS) by intrapulpal injection of 2 M KCl into upper and lower incisor pulps exposed by bone cutter in Sprague Dawley rats. The number of Fos-IR neurons was increased in the trigeminal subnucleus caudalis (Vc) and the transitional region between Vc and subnucleus interpolaris (Vi) by NS to tooth. The intradental NS raised arterial blood pressure (BP) and heart rate (HR). The number of Fos-IR neurons was also enhanced in thalamic ventral posteromedial nucleus (VPMN) and centrolateral nucleus (CLN) by NS to tooth. The intradental NS increased the number of Fos-IR neurons in the nucleus tractus solitarius (NTS) and rostral ventrolateral medulla (RVLM), hypothalamic supraoptic nucleus (SON) and paraventricular nucleus (PVN), central cardiovascular regulation centers. Ginsenosides reduced the number of c-Fos-IR increased by NS to tooth in the trigeminal Vc and thalamic VPMN and CLN. Naloxone, an opioid antagonist, did not block the effect of ginsenoside on the number of Fos-IR neurons enhanced by NS to tooth in the trigeminal Vc and thalamic VPMN and CLN. Ginsenosides ameliorated arterial BP and HR raised by NS to tooth and reduced the number of Fos-IR neurons increased by NS to tooth in the NTS, RVLM, hypothalamic SON, and PVN. These results suggest that ginsenosides have an antinociceptive effect on toothache through non-opioid system and attenuates BP and HR increased by NS to tooth.
Keyword
MeSH Terms
Figure
Reference
-
1. Sugiyo S, Takemura M, Dubner R, Ren K. Trigeminal transition zone/rostral ventromedial medulla connections and facilitation of orofacial hyperalgesia after masseter inflammation in rats. J Comp Neurol. 2005; 493:510–523. PMID: 16304628.
Article2. Kumada M, Dampney RA, Reis DJ. The trigeminal depressor response: a cardiovascular reflex originating from the trigeminal system. Brain Res. 1975; 92:485–489. PMID: 1182019.
Article3. Kumada M, Dampney RA, Reis DJ. The trigeminal depressor response: a novel vasodepressor response originating from the trigeminal system. Brain Res. 1977; 119:305–326. PMID: 830389.
Article4. Kaku T, Miyata T, Uruno T, Sako I, Kinoshita A. Chemico-pharmacological studies on saponins of Panax ginseng C. A. Meyer. II. Pharmacological part. Arzneimittelforschung. 1975; 25:539–547. PMID: 239732.5. Kim HS, Jang CG, Lee MK. Antinarcotic effects of the standardized ginseng extract G115 on morphine. Planta Med. 1990; 56:158–163. PMID: 2353061.6. Ramarao P, Bhargava HN. Antagonism of the acute pharmacological actions of morphine by panax ginseng extract. Gen Pharmacol. 1990; 21:877–880. PMID: 2279687.
Article7. Choi S, Jung SY, Rhim H, Jeong SW, Lee SM, Nah SY. Evidence that ginsenosides prevent the development of opioid tolerance at the central nervous system. Life Sci. 2000; 67:969–975. PMID: 10946856.
Article8. Choi JG, Kang SY, Kim JM, Roh DH, Yoon SY, Park JB, Lee JH, Kim HW. Antinociceptive effect of Cyperi rhizoma and corydalis tuber extracts on neuropathic pain in rats. Korean J Physiol Pharmacol. 2012; 16:387–392. PMID: 23269900.9. Mogil JS, Shin YH, McCleskey EW, Kim SC, Nah SY. Ginsenoside Rf, A trace component of ginseng root, produces antinociception in mice. Brain Res. 1998; 792:218–228. PMID: 9593902.
Article10. Rhim H, Kim H, Lee DY, Oh TH, Nah SY. Ginseng and ginsenoside Rg3, a newly identified active ingredient of ginseng, modulate Ca2+ channel currents in rat sensory neurons. Eur J Pharmacol. 2002; 436:151–158. PMID: 11858794.11. Jung JY, Yang HR, Jeong YJ, Vang MS, Park SW, Oh WM, Kim SH, Youn DH, Na CS, Kim WJ. Effects of acupuncture on c-Fos expression in brain after noxious tooth stimulation of the rat. Am J Chin Med. 2006; 34:989–1003. PMID: 17163588.
Article12. Chiu R, Boyle WJ, Meek J, Smeal T, Hunter T, Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988; 54:541–552. PMID: 3135940.
Article13. Halazonetis TD, Georgopoulos K, Greenberg ME, Leder P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell. 1988; 55:917–924. PMID: 3142692.
Article14. Chattipakorn SC, Light AR, Willcockson HH, Närhi M, Maixner W. The effect of fentanyl on c-fos expression in the trigeminal brainstem complex produced by pulpal heat stimulation in the ferret. Pain. 1999; 82:207–215. PMID: 10467925.
Article15. Fujisawa N, Terayama R, Yamaguchi D, Omura S, Yamashiro T, Sugimoto T. Fos protein-like immunoreactive neurons induced by electrical stimulation in the trigeminal sensory nuclear complex of rats with chronically injured peripheral nerve. Exp Brain Res. 2012; 219:191–201. PMID: 22456943.
Article16. Nah SY, Park HJ, McCleskey EW. A trace component of ginseng that inhibits Ca2+ channels through a pertussis toxin-sensitive G protein. Proc Natl Acad Sci U S A. 1995; 92:8739–8743. PMID: 7568008.17. Yoon SR, Nah JJ, Shin YH, Kim SK, Nam KY, Choi HS, Nah SY. Ginsenosides induce differential antinociception and inhibit substance P induced-nociceptive response in mice. Life Sci. 1998; 62:PL 319–PL 325.
Article18. Nah JJ, Choi S, Kim YH, Kim SC, Nam KY, Kim JK, Nah SY. Effect of spinally administred ginseng total saponin on capsaicin-induced pain and excitatory amino acids-induced nociceptive responses. J Ginseng Res. 1999; 23:38–43.19. Krukoff TL, Mactavish D, Jhamandas JH. Activation by hypotension of neurons in the hypothalamic paraventricular nucleus that project to the brainstem. J Comp Neurol. 1997; 385:285–296. PMID: 9268128.
Article20. Lovick TA, Li P. Integrated function of neurones in the rostral ventrolateral medulla. Prog Brain Res. 1989; 81:223–232. PMID: 2694220.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- c-Fos expression in the rat trigeminal sensory nucleus complex following tooth movement
- Effect of P. ginseng on the expression of c-Fos in the brain of Wistar rats with testosterone induced benign prostatic hyperplasia
- The Effects of Superior Cervical Ganglionectomy on C-fos Expression in Rat Brain
- Expression of Fos Protein in Brainstem Vestibular Nuclei of Rat - I. Off-axis Centripetal Acceleration -
- Effect of Hypoxia-ischemia on c-fos Expression in the Neonatal Rat Brain