Korean J Physiol Pharmacol.
2013 Jun;17(3):245-251. 10.4196/kjpp.2013.17.3.245.
Effects of Fluvastatin on the Pharmacokinetics of Repaglinide: Possible Role of CYP3A4 and P-glycoprotein Inhibition by Fluvastatin
- Affiliations
-
- 1Department of Medical Management, Chodang University, Mooan 534-701, Korea.
- 2College of Pharmacy, Chosun University, Gwangju 501-759, Korea. bjs0191@hotmail.com
- KMID: 1429305
- DOI: http://doi.org/10.4196/kjpp.2013.17.3.245
Abstract
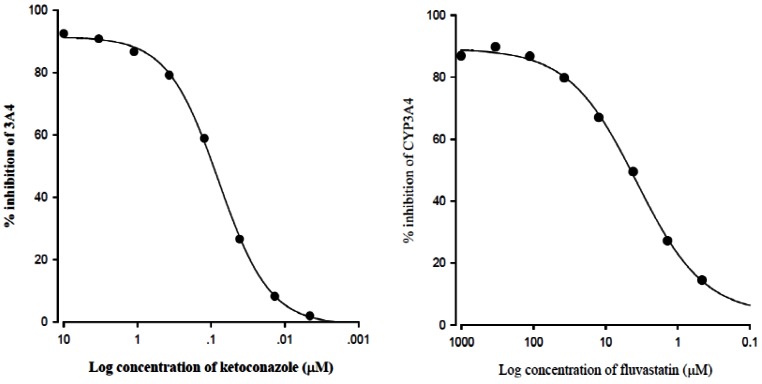
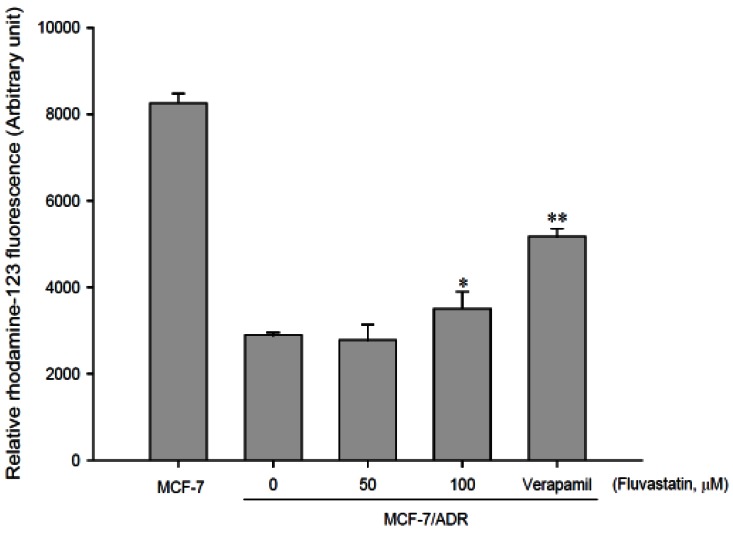
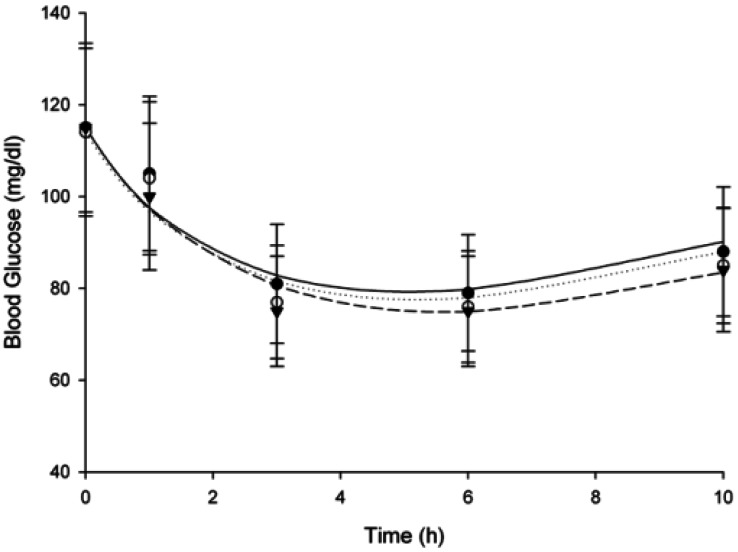
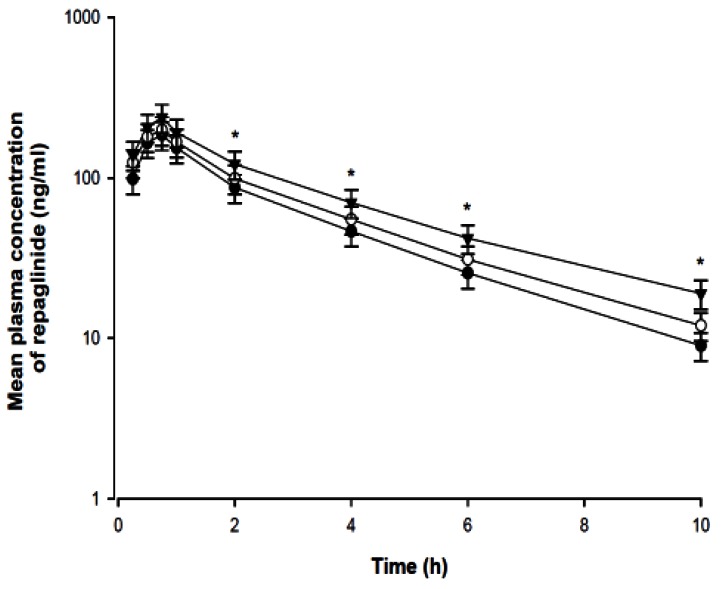
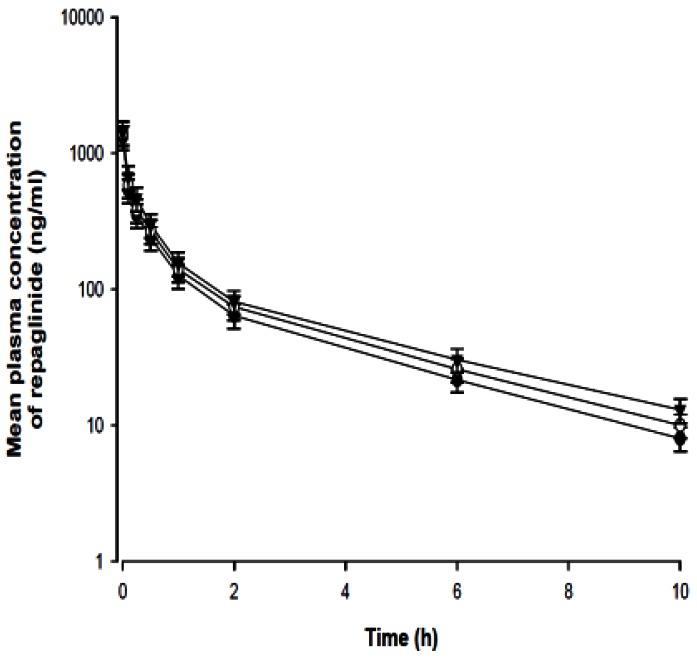
- The purpose of this study was to investigate the effects of fluvastatin on the pharmacokinetics of repaglinide in rats. The effect of fluvastatin on P-glycoprotein and CYP3A4 activity was evaluated. The pharmacokinetic parameters and blood glucose concentrations were also determined after oral and intravenous administration of repaglinide to rats in the presence and absence of fluvastatin. Fluvastatin inhibited CYP3A4 activity in a concentration-dependent manner with a 50% inhibition concentration(IC50) of 4.1 microM and P-gp activity. Compared to the oral control group, fluvastatin significantly increased the AUC and the peak plasma level of repaglinide by 45.9% and 22.7%, respectively. Fluvastatin significantly decreased the total body clearance (TBC) of repaglinide compared to the control. Fluvastatin also significantly increased the absolute bioavailability (BA) of repaglinide by 46.1% compared to the control group. Moreover, the relative BA of repaglinide was 1.14- to 1.46-fold greater than that of the control. Compared to the i.v. control, fluvastatin significantly increased the AUC0-infinity of i.v. administered repaglinide. The blood glucose concentrations showed significant differences compared to the oral controls. Fluvastatin enhanced the oral BA of repaglinide, which may be mainly attributable to the inhibition of the CYP3A4-mediated metabolism of repaglinide in the small intestine and/or liver, to the inhibition of the P-gp efflux transporter in the small intestine and/or to the reduction of TBC of repaglinide by fluvastatin. The study has raised the awareness of potential interactions during concomitant use of repaglinide with fluvastatin. Therefore, the concurrent use of repaglinide and fluvastatin may require close monitoring for potential drug interactions.
Keyword
MeSH Terms
Figure
Reference
-
1. Culy CR, Jarvis B. Repaglinide: a review of its therapeutic use in type 2 diabetes mellitus. Drugs. 2001; 61:1625–1660. PMID: 11577798.2. Bidstrup TB, Bjørnsdottir I, Sidelmann UG, Thomsen MS, Hansen KT. CYP2C8 and CYP3A4 are the principal enzymes involved in the human in vitro biotransformation of the insulin secretagogue repaglinide. Br J Clin Pharmacol. 2003; 56:305–314. PMID: 12919179.
Article3. Bauer E, Beschke K, Ebner T, Greischel A. Biotransformation of [14C] repaglinide in human, cynomolgus monkey, dog, rabbit, rat and mouse. Diabetologia. 1997; 40(Suppl 1):326–332.4. Chang C, Bahadduri PM, Polli JE, Swaan PW, Ekins S. Rapid identification of P-glycoprotein substrates and inhibitors. Drug Metab Dispos. 2006; 34:1976–1984. PMID: 16997908.
Article5. Li C, Choi DH, Choi JS. Effects of efonidipine on the pharmacokinetics and pharmacodynamics of repaglinide: possible role of CYP3A4 and P-glycoprotein inhibition by efonidipine. J Pharmacokinet Pharmacodyn. 2012; 39:99–108. PMID: 22210483.
Article6. Kajosaari LI, Niemi M, Neuvonen M, Laitila J, Neuvonen PJ, Backman JT. Cyclosporine markedly raises the plasma concentrations of repaglinide. Clin Pharmacol Ther. 2005; 78:388–399. PMID: 16198658.
Article7. Hatorp V. Clinical pharmacokinetics and pharmacodynamics of repaglinide. Clin Pharmacokinet. 2002; 41:471–483. PMID: 12083976.
Article8. Gromada J, Dissing S, Kofod H, Frøkjaer-Jensen J. Effects of the hypoglycaemic drugs repaglinide and glibenclamide on ATP-sensitive potassium-channels and cytosolic calcium levels in beta TC3 cells and rat pancreatic beta cells. Diabetologia. 1995; 38:1025–1032. PMID: 8591815.9. Kalliokoski A, Backman JT, Kurkinen KJ, Neuvonen PJ, Niemi M. Effects of gemfibrozil and atorvastatin on the pharmacokinetics of repaglinide in relation to SLCO1B1 polymorphism. Clin Pharmacol Ther. 2008; 84:488–496. PMID: 19238654.
Article10. Deslypere JP. Clinical implications of the biopharmaceutical properties of fluvastatin. Am J Cardiol. 1994; 73:12D–17D.
Article11. Cohen LH, van Leeuwen RE, van Thiel GC, van Pelt JF, Yap SH. Equally potent inhibitors of cholesterol synthesis in human hepatocytes have distinguishable effects on different cytochrome P450 enzymes. Biopharm Drug Dispos. 2000; 21:353–364. PMID: 11523064.
Article12. Plosker GL, Wagstaff AJ. Fluvastatin: a review of its pharmacology and use in the management of hyperchole sterolaemia. Drugs. 1996; 51:433–459. PMID: 8882381.13. Langtry HD, Markham A. Fluvastatin: a review of its use in lipid disorders. Drugs. 1999; 57:583–606. PMID: 10235694.14. Scripture CD, Pieper JA. Clinical pharmacokinetics of fluvastatin. Clin Pharmacokinet. 2001; 40:263–281. PMID: 11368292.
Article15. Kivistö KT, Kantola T, Neuvonen PJ. Different effects of itraconazole on the pharmacokinetics of fluvastatin and lovastatin. Br J Clin Pharmacol. 1998; 46:49–53. PMID: 9690949.
Article16. Choi JS, Piao YJ, Han HK. Pharmacokinetic interaction between fluvastatin and diltiazem in rats. Biopharm Drug Dispos. 2006; 27:437–441. PMID: 17009339.
Article17. Appel S, Rüfenacht T, Kalafsky G, Tetzloff W, Kallay Z, Hitzenberger G, Kutz K. Lack of interaction between fluvastatin and oral hypoglycemic agents in healthy subjects and in patients with non-insulin-dependent diabetes mellitus. Am J Cardiol. 1995; 76:29A–32A.18. Ruzilawati AB, Wahab MS, Imran A, Ismail Z, Gan SH. Method development and validation of repaglinide in human plasma by HPLC and its application in pharmacokinetic studies. J Pharm Biomed Anal. 2007; 43:1831–1835. PMID: 17240100.
Article19. Yoon SS, Mekalanos JJ. 2,3-butanediol synthesis and the emergence of the Vibrio cholerae El Tor biotype. Infect Immun. 2006; 74:6547–6556. PMID: 17015461.20. Crespi CL, Miller VP, Penman BW. Microtiter plate assays for inhibition of human, drug-metabolizing cytochromes P450. Anal Biochem. 1997; 248:188–190. PMID: 9177742.
Article21. Park JK, Kim SP, Song DK. Ameliorating effects of sulfonylurea drugs on insulin resistance in Otsuka long-evans Tokushima Fatty rats. Korean J Physiol Pharmacol. 2008; 12:7–12. PMID: 20157388.
Article22. Mason RP. A rationale for combined therapy with a calcium channel blocker and a statin: evaluation of basic and clinical evidence. Curr Drug Targets Cardiovasc Haematol Disord. 2005; 5:489–501. PMID: 16503869.
Article23. Cummins CL, Jacobsen W, Benet LZ. Unmasking the dynamic interplay between intestinal P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002; 300:1036–1045. PMID: 11861813.
Article24. Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000; 57:1439–1443. PMID: 11030795.
Article25. Sutherland L, Ebner T, Burchell B. The expression of UDP-glucuronosyltransferases of the UGT1 family in human liver and kidney and in response to drugs. Biochem Pharmacol. 1993; 45:295–301. PMID: 8435089.
Article26. Turgeon D, Carrier JS, Lévesque E, Hum DW, Bélanger A. Relative enzymatic activity, protein stability, and tissue distribution of human steroid-metabolizing UGT2B subfamily members. Endocrinology. 2001; 142:778–787. PMID: 11159850.27. Watkins PB. The barrier function of CYP3A4 and P-glycoprotein in the small bowel. Adv Drug Deliv Rev. 1997; 27:161–170. PMID: 10837556.
Article28. Niemi M, Neuvonen PJ, Kivistö KT. The cytochrome P4503A4 inhibitor clarithromycin increases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2001; 70:58–65. PMID: 11452245.
Article29. Wacher VJ, Silverman JA, Zhang Y, Benet LZ. Role of P-glycoprotein and cytochrome P450 3A in limiting oral absorption of peptides and peptidomimetics. J Pharm Sci. 1998; 87:1322–1330. PMID: 9811484.
Article30. Kelly PA, Wang H, Napoli KL, Kahan BD, Strobel HW. Metabolism of cyclosporine by cytochromes P450 3A9 and 3A4. Eur J Drug Metab Pharmacokinet. 1999; 24:321–328. PMID: 10892895.
Article31. Lee CK, Choi JS. Effects of silibinin, inhibitor of CYP3A4 and P-glycoprotein in vitro, on the pharmacokinetics of paclitaxel after oral and intravenous administration in rats. Pharmacology. 2010; 85:350–356. PMID: 20523105.
Article32. Hong SP, Choi DH, Choi JS. Effects of resveratrol on the pharmacokinetics of diltiazem and its major metabolite, desacetyldiltiazem, in rats. Cardiovasc Ther. 2008; 26:269–275. PMID: 19035878.
Article33. Choi DH, Choi JS, Li C, Choi JS. Effects of simvastatin on the pharmacokinetics of diltiazem and its main metabolite, desacetyldiltiazem, after oral and intravenous administration in rats: possible role of P-glycoprotein and CYP3A4 inhibition by simvastatin. Pharmacol Rep. 2011; 63:1574–1582. PMID: 22358108.34. Kivistö KT, Bookjans G, Fromm MF, Griese EU, Münzel P, Kroemer HK. Expression of CYP3A4, CYP3A5 and CYP3A7 in human duodenal tissue. Br J Clin Pharmacol. 1996; 42:387–389. PMID: 8877031.35. Zhang QY, Dunbar D, Ostrowska A, Zeisloft S, Yang J, Kaminsky LS. Characterization of human small intestinal cytochromes P-450. Drug Metab Dispos. 1999; 27:804–809. PMID: 10383924.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fatal Rhabdomyolysis in a Patient with Liver Cirrhosis after Switching from Simvastatin to Fluvastatin
- Efficacy of Fluvastatin in Patients with Hypercholesterolemia
- Fluvastatin inhibits advanced glycation end products-induced proliferation, migration, and extracellular matrix accumulation in vascular smooth muscle cells by targeting connective tissue growth factor
- Effect of post-treatment fluvastatin for hemorrhagic shock in rats
- Rhabdomyolysis in a Cyclosporine-treated Renal Transplant Recipient Who Received Atorvastatin as Replacement for Fluvastatin