Korean J Physiol Pharmacol.
2013 Jun;17(3):203-208. 10.4196/kjpp.2013.17.3.203.
5,8-Dimethoxy-2-Nonylamino-Naphthalene-1,4-Dione Inhibits Vascular Smooth Muscle Cell Proliferation by Blocking Autophosphorylation of PDGF-Receptor beta
- Affiliations
-
- 1Department of Pharmacology, College of Pharmacy, Chungnam National University, Daejeon 305-764, Korea. cm8r@cnu.ac.kr
- 2Institute of Drug Research & Development, Chungnam National University, Daejeon 305-764, Korea.
- 3Department of Medicinal Chemistry, College of Pharmacy, Chungnam National University, Daejeon 305-764, Korea.
- KMID: 1429299
- DOI: http://doi.org/10.4196/kjpp.2013.17.3.203
Abstract
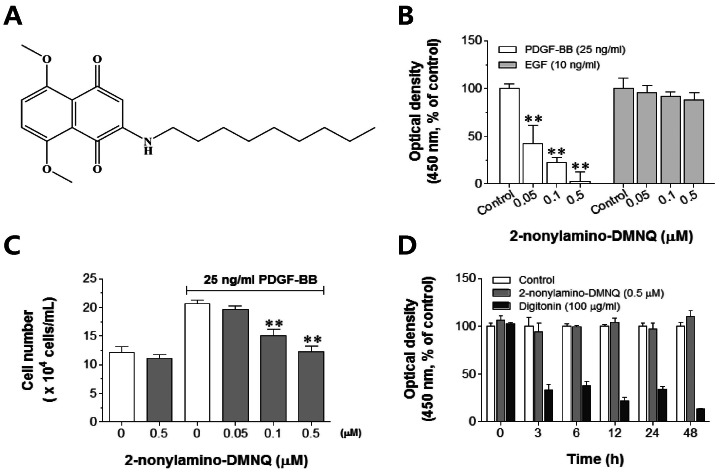
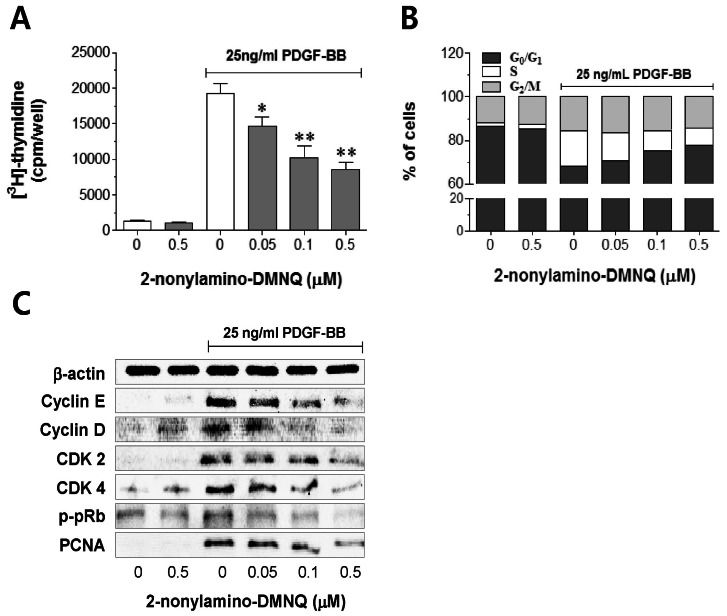
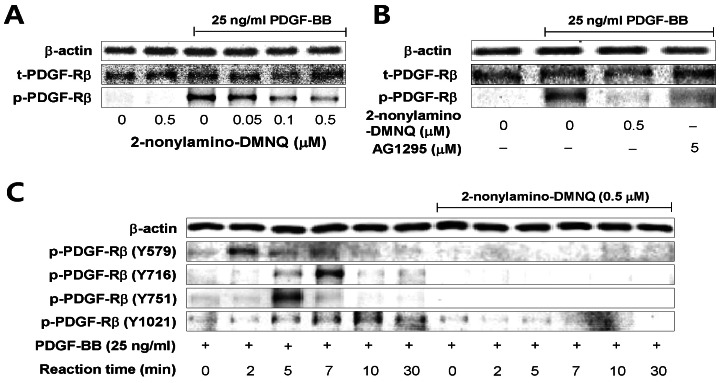
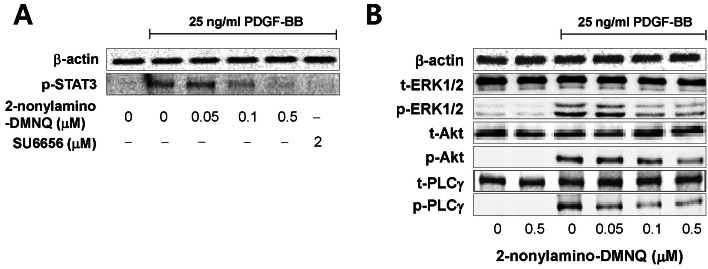
- As the abnormal proliferation of vascular smooth muscle cells (VSMCs) plays a critical role in the development of atherosclerosis and vascular restenosis, a candidate drug with antiproliferative properties is needed. We investigated the antiproliferative action and underlying mechanism of a newly synthesized naphthoquinone derivative, 5,8-dimethoxy-2-nonylamino-naphthalene-1,4-dione (2-nonylamino-DMNQ), using VSMCs treated with platelet-derived growth factor (PDGF). 2-Nonylamino-DMNQ inhibited proliferation and cell number of VSMCs induced by PDGF, but not epidermal growth factor (EGF), in a concentration-dependent manner without any cytotoxicity. This derivative suppressed PDGF-induced [3H]-thymidine incorporation, cell cycle progression from G0/G1 to S phase, and the phosphorylation of phosphor-retinoblastoma protein (pRb) as well as the expression of cyclin E/D, cyclin-dependent kinase (CDK) 2/4, and proliferating cell nuclear antigen (PCNA). Importantly, 2-nonylamino-DMNQ inhibited the phosphorylation of PDGF receptorbeta(PDGF-Rbeta) enhanced by PDGF at Tyr579, Tyr716, Tyr751, and Tyr1021 residues. Subsequently, 2-nonylamino-DMNQ inhibited PDGF-induced phosphorylation of STAT3, ERK1/2, Akt, and PLCgamma1. Therefore, our results indicate that 2-nonylamino-DMNQ inhibits PDGF-induced VSMC proliferation by blocking PDGF-Rbeta autophosphorylation, and subsequently PDGF-Rbeta-mediated downstream signaling pathways.
Keyword
MeSH Terms
-
Atherosclerosis
Cardiovascular Diseases
Cell Count
Cell Cycle
Cell Proliferation
Cyclins
Epidermal Growth Factor
Muscle, Smooth, Vascular
Phosphorylation
Phosphotransferases
Platelet-Derived Growth Factor
Proliferating Cell Nuclear Antigen
S Phase
Cyclins
Epidermal Growth Factor
Phosphotransferases
Platelet-Derived Growth Factor
Proliferating Cell Nuclear Antigen
Figure
Reference
-
1. Bailey SR. Coronary restenosis: a review of current insights and therapies. Catheter Cardiovasc Interv. 2002; 55:265–271. PMID: 11835664.
Article2. Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995; 57:791–804. PMID: 7778883.
Article3. Shimokado K, Yokota T, Kosaka C, Zen K, Sasaguri T, Masuda J, Ogata J. Protein tyrosine kinase inhibitors inhibit both proliferation and chemotaxis of vascular smooth muscle cells. Ann N Y Acad Sci. 1995; 748:171–175. PMID: 7695163.4. Claesson-Welsh L. Platelet-derived growth factor receptor signals. J Biol Chem. 1994; 269:32023–32026. PMID: 7798193.
Article5. Downward J. Signal transduction. New exchange, new target. Nature. 1998; 396:416–417. PMID: 9853744.6. Higaki M, Shimokado K. Phosphatidylinositol 3-kinase is required for growth factor-induced amino acid uptake by vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999; 19:2127–2132. PMID: 10479654.
Article7. Sachinidis A, Kraus R, Seul C, Meyer zu, Schulte K, Ko Y, Hoppe J, Vetter H. Gangliosides GM1, GM2 and GM3 inhibit the platelet-derived growth factor-induced signalling transduction pathway in vascular smooth muscle cells by different mechanisms. Eur J Cell Biol. 1996; 71:79–88. PMID: 8884181.8. Kingsley K, Huff JL, Rust WL, Carroll K, Martinez AM, Fitchmun M, Plopper GE. ERK1/2 mediates PDGF-BB stimulated vascular smooth muscle cell proliferation and migration on laminin-5. Biochem Biophys Res Commun. 2002; 293:1000–1006. PMID: 12051759.
Article9. Chlebowski RT, Dietrich M, Akman S, Block JB. Vitamin K3 inhibition of malignant murine cell growth and human tumor colony formation. Cancer Treat Rep. 1985; 69:527–532. PMID: 4005875.10. Kar S, Carr BI. Growth inhibition and protein tyrosine phosphorylation in MCF 7 breast cancer cells by a novel K vitamin. J Cell Physiol. 2000; 185:386–393. PMID: 11056008.
Article11. Ko FN, Sheu SJ, Liu YM, Huang TF, Teng CM. Inhibition of rabbit platelet aggregation by 1,4-naphthoquinones. Thromb Res. 1990; 57:453–463. PMID: 2156351.
Article12. Rodriguez S, Wolfender JL, Hakizamungu E, Hostettmann K. An antifungal naphthoquinone, xanthones and secoiridoids from Swertia calycina. Planta Med. 1995; 61:362–364. PMID: 7480185.13. Shen AY, Huang MH, Teng CM, Wang JS. Inhibition of 2-p-mercaptophenyl-1,4-naphthoquinone on human platelet function. Life Sci. 1999; 65:45–53. PMID: 10403492.14. Gant TW, Rao DN, Mason RP, Cohen GM. Redox cycling and sulphydryl arylation; their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes. Chem Biol Interact. 1988; 65:157–173. PMID: 2835188.
Article15. Shi M, Gozal E, Choy HA, Forman HJ. Extracellular glutathione and gamma-glutamyl transpeptidase prevent H2O2-induced injury by 2,3-dimethoxy-1,4-naphthoquinone. Free Radic Biol Med. 1993; 15:57–67. PMID: 8103030.16. Thor H, Smith MT, Hartzell P, Bellomo G, Jewell SA, Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982; 257:12419–12425. PMID: 6181068.
Article17. Liu RM, Shi MM, Giulivi C, Forman HJ. Quinones increase gamma-glutamyl transpeptidase expression by multiple mechanisms in rat lung epithelial cells. Am J Physiol. 1998; 274:L330–L336. PMID: 9530167.18. Shi MM, Kugelman A, Iwamoto T, Tian L, Forman HJ. Quinone-induced oxidative stress elevates glutathione and induces γ-glutamylcysteine synthetase activity in rat lung epithelial L2 cells. J Biol Chem. 1994; 269:26512–26517. PMID: 7929374.19. Lee JJ, Zhang WY, Yi H, Kim Y, Kim IS, Shen GN, Song GY, Myung CS. Anti-proliferative actions of 2-decylamino-5,8-dimethoxy-1,4-naphthoquinone in vascular smooth muscle cells. Biochem Biophys Res Commun. 2011; 411:213–218. PMID: 21726525.
Article20. Chamley JH, Campbell GR, McConnell JD, Gröschel-Stewart U. Comparison of vascular smooth muscle cells from adult human, monkey and rabbit in primary culture and in subculture. Cell Tissue Res. 1977; 177:503–522. PMID: 402216.
Article21. Lee JJ, Yu JY, Zhang WY, Kim TJ, Lim Y, Kwon JS, Kim DW, Myung CS, Yun YP. Inhibitory effect of fenofibrate on neointima hyperplasia via G0/G1 arrest of cell proliferation. Eur J Pharmacol. 2011; 650:342–349. PMID: 21040719.22. Lee JJ, Yi H, Kim IS, Kim Y, Nhiem NX, Kim YH, Myung CS. (2S)-naringenin from Typha angustata inhibits vascular smooth muscle cell proliferation via a G0/G1 arrest. J Ethnopharmacol. 2012; 139:873–878. PMID: 22212500.23. Banai S, Wolf Y, Golomb G, Pearle A, Waltenberger J, Fishbein I, Schneider A, Gazit A, Perez L, Huber R, Lazarovichi G, Rabinovich L, Levitzki A, Gertz SD. PDGF-receptor tyrosine kinase blocker AG1295 selectively attenuates smooth muscle cell growth in vitro and reduces neointimal formation after balloon angioplasty in swine. Circulation. 1998; 97:1960–1969. PMID: 9609090.24. Schwaiberger AV, Heiss EH, Cabaravdic M, Oberan T, Zaujec J, Schachner D, Uhrin P, Atanasov AG, Breuss JM, Binder BR, Dirsch VM. Indirubin-3'-monoxime blocks vascular smooth muscle cell proliferation by inhibition of signal transducer and activator of transcription 3 signaling and reduces neointima formation in vivo. Arterioscler Thromb Vasc Biol. 2010; 30:2475–2481. PMID: 20847306.25. Fang F, Newport JW. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell. 1991; 66:731–742. PMID: 1652371.
Article26. Moore GD, Lear SC, Wills-Frank LA, Martin AW, Snyder JW, Helm CW. Differential expression of cdk inhibitors p16, p21cip1, p27kip1, and cyclin E in cervical cytological smears prepared by the ThinPrep method. Diagn Cytopathol. 2005; 32:82–87. PMID: 15637682.
Article27. Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002; 8:1249–1256. PMID: 12411952.
Article28. Tomita H, Osanai T, Toki T, Maeda N, Murakami R, Chen Z, Yamabe H, Osawa H, Yasujima M, Okumura K. Roxithromycin is an inhibitor of human coronary artery smooth muscle cells proliferation: a potential ability to prevent coronary heart disease. Atherosclerosis. 2005; 182:87–95. PMID: 16115478.
Article29. Beier I, Düsing R, Vetter H, Schmitz U. Epidermal growth factor stimulates Rac1 and p21-activated kinase in vascular smooth muscle cells. Atherosclerosis. 2008; 196:92–97. PMID: 17350025.
Article30. Mori S, Rönnstrand L, Yokote K, Engström A, Courtneidge SA, Claesson-Welsh L, Heldin CH. Identification of two juxtamembrane autophosphorylation sites in the PDGF β-receptor; involvement in the interaction with Src family tyrosine kinases. EMBO J. 1993; 12:2257–2264. PMID: 7685273.31. Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM, Irby R, Yeatman T, Courtneidge SA, Jove R. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci U S A. 2001; 98:7319–7324. PMID: 11404481.
Article32. Arvidsson AK, Rupp E, Nånberg E, Downward J, Rönnstrand L, Wennström S, Schlessinger J, Heldin CH, Claesson-Welsh L. Tyr-716 in the platelet-derived growth factor β-receptor kinase insert is involved in GRB2 binding and Ras activation. Mol Cell Biol. 1994; 14:6715–6726. PMID: 7935391.33. Dance M, Montagner A, Salles JP, Yart A, Raynal P. The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal. 2008; 20:453–459. PMID: 17993263.
Article34. Panayotou G, Bax B, Gout I, Federwisch M, Wroblowski B, Dhand R, Fry MJ, Blundell TL, Wollmer A, Waterfield MD. Interaction of the p85 subunit of PI 3-kinase and its N-terminal SH2 domain with a PDGF receptor phosphorylation site: structural features and analysis of conformational changes. EMBO J. 1992; 11:4261–4272. PMID: 1330535.
Article35. Kashishian A, Kazlauskas A, Cooper JA. Phosphorylation sites in the PDGF receptor with different specificities for binding GAP and PI3 kinase in vivo. EMBO J. 1992; 11:1373–1382. PMID: 1314164.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mechanisms Involved in the Inhibitory Effects of Mycophenolic Acid on the PDGF-induced Proliferation of Vascular Smooth Muscle Cells
- Influence of Endothelin-1 on Cultured Vascular Smooth Muscle Cell Proliferation
- Effect of Carvedilol on the Growth of Vascular Smooth Muscle Cells
- Dexamethasone Attenuates PDGF- and TGF-beta-enhanced Vascular Endothelial Growth Factor Production in Cultured Human Bronchial Smooth Muscle Cells
- Effect of Carvedilol on Human Vascular Smooth Muscle Cell Proliferation and Its Signal Transduction