Korean J Physiol Pharmacol.
2013 Jun;17(3):189-195. 10.4196/kjpp.2013.17.3.189.
Altered APP Carboxyl-Terminal Processing Under Ferrous Iron Treatment in PC12 Cells
- Affiliations
-
- 1Department of Biomedical Engineering, College of Health Science, Yonsei University, Wonju 220-710, Korea. yooym@yonsei.ac.kr
- KMID: 1429297
- DOI: http://doi.org/10.4196/kjpp.2013.17.3.189
Abstract
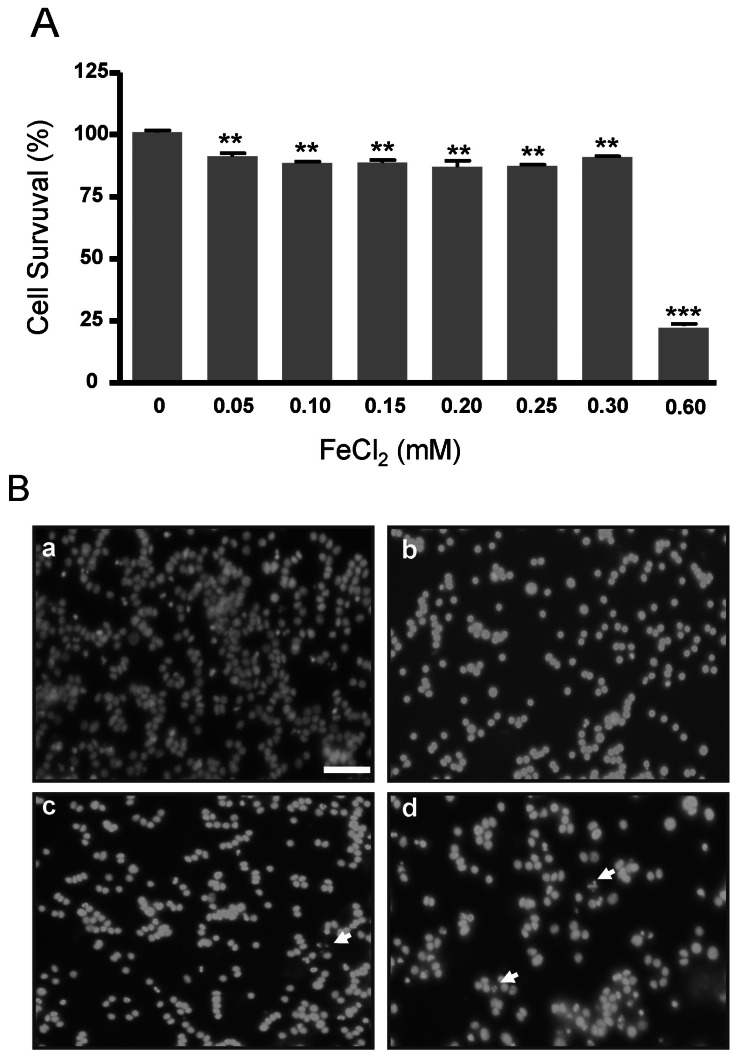
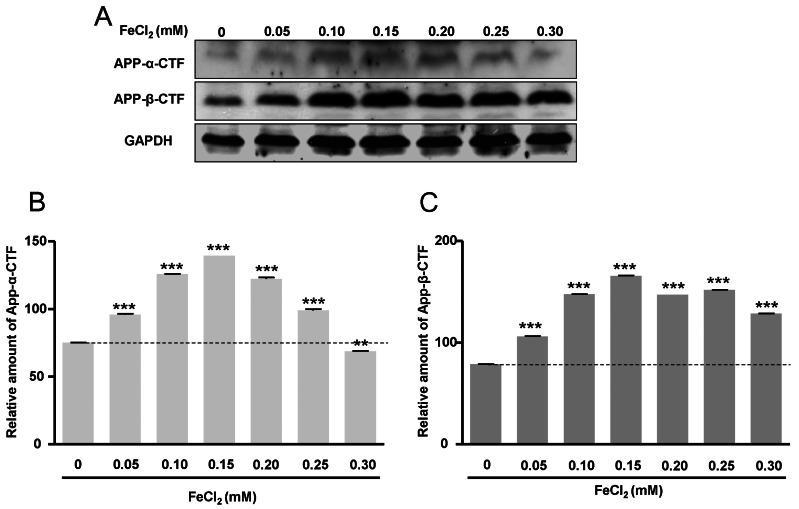
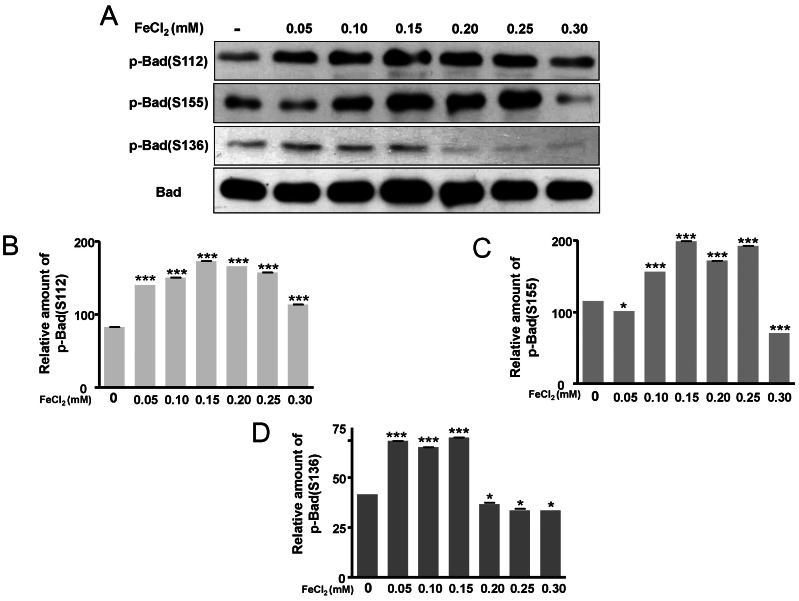
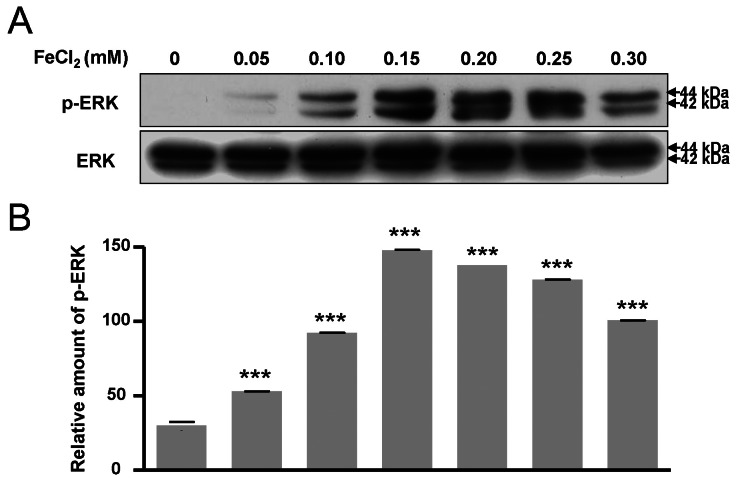
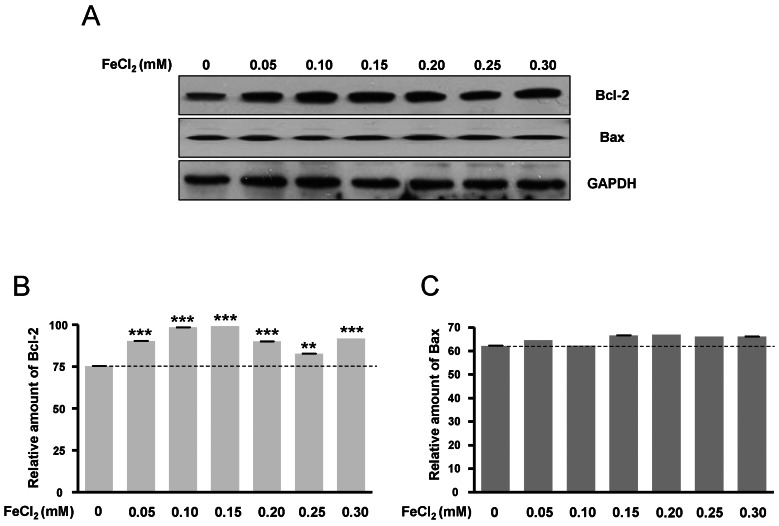
- Amyloid-beta peptide (Abeta), generated by proteolytic cleavage of the amyloid precursor protein (APP), plays a pivotal role in the pathogenesis of Alzheimer's disease (AD). The key step in the generation of Abeta is cleavage of APP by beta-site APP-cleaving enzyme 1 (BACE1). Levels of BACE1 are increased in vulnerable regions of the AD brain, but the underlying mechanism is unknown. In the present study, we reported the effects of ferrous ions at subtoxic concentrations on the mRNA levels of BACE1 and a-disintegrin-and-metalloproteinase 10 (ADAM10) in PC12 cells and the cell responses to ferrous ions. The cell survival in PC12 cells significantly decreased with 0 to 0.3 mM FeCl2, with 0.6 mM FeCl2 treatment resulting in significant reductions by about 75%. 4,6-diamidino-2-phenylindole (DAPI) staining showed that the nuclei appeared fragmented in 0.2 and 0.3 mM FeCl2. APP-alpha-carboxyl terminal fragment (APP-alpha-CTF) associations with ADAM10 and APP-beta-CTF with BACE1 were increased. Levels of ADAM10 and BACE1 mRNA increased in response to the concentrations of 0.25 mM, respectively. In addition, p-ERK and p-Bad (S112, S155) expressions were increased, suggesting that APP-CTF formation is related to ADAM10/BACE1 expression. Levels of Bcl-2 protein were increased, but significant changes were not observed in the expression of Bax. These data suggest that ion-induced enhanced expression of AMDA10/BACE1 could be one of the causes for APP-alpha/beta-CTF activation.
Keyword
MeSH Terms
Figure
Reference
-
1. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002; 297:353–356. PMID: 12130773.
Article2. Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004; 430:631–639. PMID: 15295589.
Article3. Pietrzik C, Behl C. Concepts for the treatment of Alzheimer's disease: molecular mechanisms and clinical application. Int J Exp Pathol. 2005; 86:173–185. PMID: 15910551.
Article4. Praticò D. Evidence of oxidative stress in Alzheimer's disease brain and antioxidant therapy: lights and shadows. Ann N Y Acad Sci. 2008; 1147:70–78. PMID: 19076432.5. Maynard CJ, Cappai R, Volitakis I, Cherny RA, White AR, Beyreuther K, Masters CL, Bush AI, Li QX. Overexpression of Alzheimer's disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J Biol Chem. 2002; 277:44670–44676. PMID: 12215434.6. Pallàs M, Camins A. Molecular and biochemical features in Alzheimer's disease. Curr Pharm Des. 2006; 12:4389–4408. PMID: 17105434.7. Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci. 1998; 158:47–52. PMID: 9667777.
Article8. Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004; 5:863–873. PMID: 15496864.
Article9. Honda K, Smith MA, Zhu X, Baus D, Merrick WC, Tartakoff AM, Hattier T, Harris PL, Siedlak SL, Fujioka H, Liu Q, Moreira PI, Miller FP, Nunomura A, Shimohama S, Perry G. Ribosomal RNA in Alzheimer disease is oxidized by bound redox-active iron. J Biol Chem. 2005; 280:20978–20986. PMID: 15767256.
Article10. Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958; 3:41–51. PMID: 13611557.
Article11. Bartzokis G, Beckson M, Hance DB, Marx P, Foster JA, Marder SR. MR evaluation of age-related increase of brain iron in young adult and older normal males. Magn Reson Imaging. 1997; 15:29–35. PMID: 9084022.
Article12. Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging. 2004; 25:5–18. PMID: 14675724.
Article13. Bartzokis G, Tishler TA, Lu PH, Villablanca P, Altshuler LL, Carter M, Huang D, Edwards N, Mintz J. Brain ferritin iron may influence age-and gender-related risks of neurodegeneration. Neurobiol Aging. 2007; 28:414–423. PMID: 16563566.14. Zhu X, Castellani RJ, Takeda A, Nunomura A, Atwood CS, Perry G, Smith MA. Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: the 'two hit' hypothesis. Mech Ageing Dev. 2001; 123:39–46. PMID: 11640950.
Article15. Li G, Yang Q, Krishnan S, Alexander EA, Borkan SC, Schwartz JH. A novel cellular survival factor--the B2 subunit of vacuolar H+-ATPase inhibits apoptosis. Cell Death Differ. 2006; 13:2109–2117. PMID: 16710359.16. Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999; 19:5800–5810. PMID: 10409766.17. di Mari JF, Davis R, Safirstein RL. MAPK activation determines renal epithelial cell survival during oxidative injury. Am J Physiol. 1999; 277:F195–F203. PMID: 10444573.18. Arany I, Megyesi JK, Kaneto H, Tanaka S, Safirstein RL. Activation of ERK or inhibition of JNK ameliorates H(2)O(2) cytotoxicity in mouse renal proximal tubule cells. Kidney Int. 2004; 65:1231–1239. PMID: 15086462.
Article19. Gottlieb RA. Role of mitochondria in apoptosis. Crit Rev Eukaryot Gene Expr. 2000; 10:231–239. PMID: 11272466.
Article20. Howard S, Bottino C, Brooke S, Cheng E, Giffard RG, Sapolsky R. Neuroprotective effects of bcl-2 overexpression in hippocampal cultures: interactions with pathways of oxidative damage. J Neurochem. 2002; 83:914–923. PMID: 12421364.
Article21. Starkov AA, Polster BM, Fiskum G. Regulation of hydrogen peroxide production by brain mitochondria by calcium and Bax. J Neurochem. 2002; 83:220–228. PMID: 12358746.
Article22. Zacchetti D, Chieregatti E, Bettegazzi B, Mihailovich M, Sousa VL, Grohovaz F, Meldolesi J. BACE1 expression and activity: relevance in Alzheimer's disease. Neurodegener Dis. 2007; 4:117–126. PMID: 17596706.
Article23. Mohajeri MH, Saini KD, Nitsch RM. Transgenic BACE expression in mouse neurons accelerates amyloid plaque pathology. J Neural Transm. 2004; 111:413–425. PMID: 14991462.
Article24. Nakamura M, Shishido N, Nunomura A, Smith MA, Perry G, Hayashi Y, Nakayama K, Hayashi T. Three histidine residues of amyloid-beta peptide control the redox activity of copper and iron. Biochemistry. 2007; 46:12737–12743. PMID: 17929832.25. Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, Freedman SB, Frigon NL, Games D, Hu K, Johnson-Wood K, Kappenman KE, Kawabe TT, Kola I, Kuehn R, Lee M, Liu W, Motter R, Nichols NF, Power M, Robertson DW, Schenk D, Schoor M, Shopp GM, Shuck ME, Sinha S, Svensson KA, Tatsuno G, Tintrup H, Wijsman J, Wright S, McConlogue L. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer's disease therapeutics. Hum Mol Genet. 2001; 10:1317–1324. PMID: 11406613.
Article26. Lee EB, Skovronsky DM, Abtahian F, Doms RW, Lee VM. Secretion and intracellular generation of truncated Abeta in beta-site amyloid-beta precursor protein-cleaving enzyme expressing human neurons. J Biol Chem. 2003; 278:4458–4466. PMID: 12480937.27. Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL, McCord JM, Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987; 107:526–545. PMID: 3307585.
Article28. Smith MA, Sayre LM, Monnier VM, Perry G. Radical AGEing in Alzheimer's disease. Trends Neurosci. 1995; 18:172–176. PMID: 7778188.
Article29. Avramovich-Tirosh Y, Amit T, Bar-Am O, Weinreb O, Youdim MB. Physiological and pathological aspects of Abeta in iron homeostasis via 5'UTR in the APP mRNA and the therapeutic use of iron-chelators. BMC Neurosci. 2008; 9(Suppl 2):S2. PMID: 19090990.
Article30. Smith MA, Nunomura A, Zhu X, Takeda A, Perry G. Metabolic, metallic, and mitotic sources of oxidative stress in Alzheimer disease. Antioxid Redox Signal. 2000; 2:413–420. PMID: 11229355.
Article31. Atwood CS, Obrenovich ME, Liu T, Chan H, Perry G, Smith MA, Martins RN. Amyloid-beta: a chameleon walking in two worlds: a review of the trophic and toxic properties of amyloid-beta. Brain Res Brain Res Rev. 2003; 43:1–16. PMID: 14499458.32. Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron. 1993; 10:243–254. PMID: 8094963.33. Furukawa K, Sopher BL, Rydel RE, Begley JG, Pham DG, Martin GM, Fox M, Mattson MP. Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J Neurochem. 1996; 67:1882–1896. PMID: 8863493.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mercury induced the Accumulation of Amyloid Beta (Abeta) in PC12 Cells: The Role of Production and Degradation of Abeta
- Iron Supplementation in a Girl with Attention-Deficit Hyperactivity Disorder
- Pathogenesis of Alzheimer's Dementia
- Effect of the mutation in the carboxyl-terminal processing site of the hepatitis B virus core antigen on the HBeAg secretion
- Clinical Eeffects of Ferrocholinate in the Iron Deficiency Anemia of Children