J Korean Soc Magn Reson Med.
2013 Mar;17(1):19-25. 10.13104/jksmrm.2013.17.1.19.
Evaluation of Renal Oxygenation in Normal Korean Volunteers Using 3.0 T Blood Oxygen Level-Dependent MRI
- Affiliations
-
- 1Department of Radiology, Seoul National University Bundang Hospital, Korea. hakjlee@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Korea.
- KMID: 1426747
- DOI: http://doi.org/10.13104/jksmrm.2013.17.1.19
Abstract
- PURPOSE
Renal blood oxygen level-dependent (BOLD) MRI has been used in the evaluation of renal oxygenation. We tried to provide the normal R2* value of the human kidney with 3.0 T, and evaluated the differences in R2* values according to gender and location.
MATERIALS AND METHODS
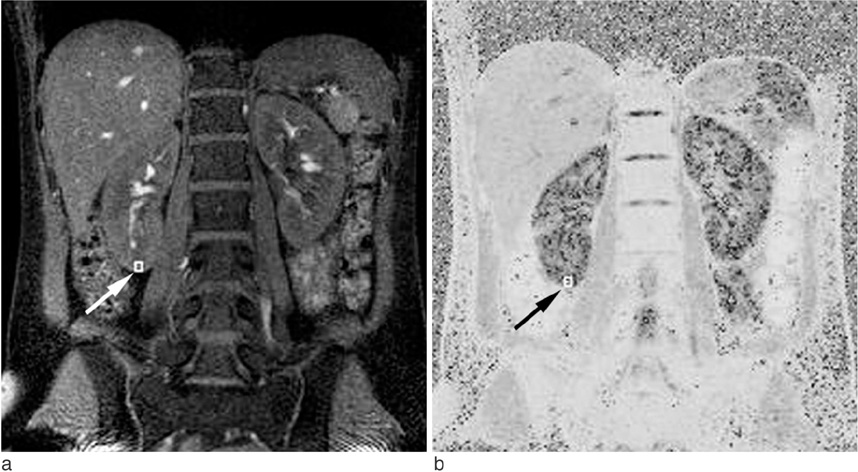
Twenty-four healthy volunteers underwent BOLD MRI at 3.0 T. Multi gradient echo-echo planar imaging sequence with seventeen echoes was used. After generation of the T2* map, the R2* was calculated. The statistical differences in R2* values between the cortex and medulla, males and females, and the right and left kidney were analyzed. The regional differences of R2* within the both kidneys were evaluated respectively.
RESULTS
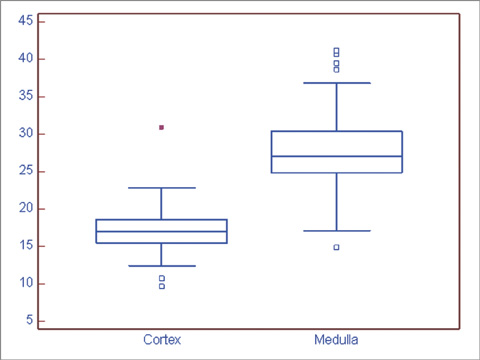
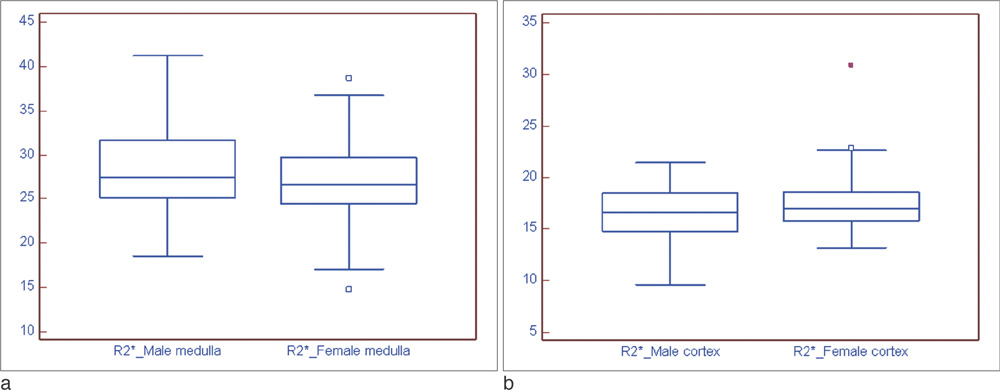
BOLD MRI was successful in all participants. No gross artifact interfered with R2* measurement. The mean R2* at 3.0 T was 17.1 +/- 2.60 s-1 in the cortex and 27.7 +/- 4.83 s-1 in the medulla (p < 0.001). The R2* value in the medulla was significantly higher in the male than female volunteers (p = 0.025). There were no statistical differences of R2* according to the side and location in the kidney (p = 0.197).
CONCLUSION
Renal BOLD MRI can be efficiently performed with 3.0 T MRI. Renal medullary hypoxia is present in normal volunteers. Our results may be used as reference values in the evaluation of pathologic conditions using BOLD MRI.
Keyword
Figure
Reference
-
1. Kone BC. A 'BOLD' new approach to renal oxygen economy. Circulation. 1996; 94:3067–3068.2. Epstein FH, Agmon Y, Brezis M. Physiology of renal hypoxia. Ann N Y Acad Sci. 1994; 718:72–81. discussion 81-72.3. Heyman SN, Brezis M, Reubinoff CA, et al. Acute renal failure with selective medullary injury in the rat. J Clin Invest. 1988; 82:401–412.4. Cowley AW Jr, Mattson DL, Lu S, Roman RJ. The renal medulla and hypertension. Hypertension. 1995; 25:663–673.5. Ries M, Basseau F, Tyndal B, et al. Renal diffusion and bold MRI in experimental diabetic nephropathy. Blood oxygen level-dependent. J Magn Reson Imaging. 2003; 17:104–113.6. Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990; 87:9868–9872.7. Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with bold MRI. Circulation. 1996; 94:3271–3275.8. Ogawa S, Menon RS, Tank DW, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. A comparison of signal characteristics with a biophysical model. Biophys J. 1993; 64:803–812.9. Li LP, Vu AT, Li BS, Dunkle E, Prasad PV. Evaluation of intrarenal oxygenation by bold MRI at 3.0 T. J Magn Reson Imaging. 2004; 20:901–904.10. Tumkur S, Vu A, Li L, Prasad PV. Evaluation of intrarenal oxygenation at 3.0 T using 3-dimensional multiple gradientrecalled echo sequence. Invest Radiol. 2006; 41:181–184.11. Prasad PV, Chen Q, Goldfarb JW, Epstein FH, Edelman RR. Breath-hold R2* mapping with a multiple gradient-recalled echo sequence: application to the evaluation of intrarenal oxygenation. J Magn Reson Imaging. 1997; 7:1163–1165.12. Prasad PV, Priatna A, Spokes K, Epstein FH. Changes in intrarenal oxygenation as evaluated by bold MRI in a rat kidney model for radiocontrast nephropathy. J Magn Reson Imaging. 2001; 13:744–747.13. Simon-Zoula SC, Hofmann L, et al. Non-invasive monitoring of renal oxygenation using bold-MRI: a reproducibility study. NMR Biomed. 2006; 19:84–89.14. Sadowski EA, Fain SB, Alford SK, et al. Assessment of acute renal transplant rejection with blood oxygen level-dependent MR imaging: initial experience. Radiology. 2005; 236:911–919.15. Li LP, Storey P, Pierchala L, Li W, Polzin J, Prasad PV. Evaluation of the reproducibility of intrarenal R2* and delta R2* measurements following administration of furosemide and during waterload. J Magn Reson Imaging. 2004; 19:610–616.16. Hofmann L, Simon-Zoula S, Nowak A, et al. Bold-MRI for the assessment of renal oxygenation in humans: acute effect of nephrotoxic xenobiotics. Kidney Int. 2006; 70:144–150.17. Tumkur SM, Vu AT, Li LP, Pierchala L, Prasad PV. Evaluation of intra-renal oxygenation during water diuresis: a timeresolved study using bold MRI. Kidney Int. 2006; 70:139–143.18. Prasad PV, Epstein FH. Changes in renal medullary PO2 during water diuresis as evaluated by blood oxygenation level-dependent magnetic resonance imaging: effects of aging and cyclooxygenase inhibition. Kidney Int. 1999; 55:294–298.19. Pechère-Bertschi A, Maillard M, Stalder H, et al. Renal hemodynamic and tubular responses to salt in women using oral contraceptives. Kidney Int. 2003; 64:1374–1380.20. Ji H, Pesce C, Zheng W, et al. Sex differences in renal injury and nitric oxide production in renal wrap hypertension. Am J Physiol Heart Circ Physiol. 2005; 288:H43–H47.21. Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000; 11:319–329.22. Cook CI, Yu BP. Iron accumulation in aging: modulation by dietary restriction. Mech Ageing Dev. 1998; 102:1–13.23. Chandarana H, Lee VS. Renal functional MRI: are we ready for clinical application? AJR Am J Roentgenol. 2009; 192:1550–1557.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroactivation studies using Functional Brain MRI
- Evaluation of Renal Oxygenation Level Changes after Water Loading Using Susceptibility-Weighted Imaging and T2* Mapping
- Quantitative Analysis of Susceptibility Effects in TRFGE and CGE Sequences for Functional MRI
- Transperitoneal Oxygenation with Lactated Ringer's Solution
- Functional Magnetic Resonance Imaging with Arterial Spin Labeling: Techniques and Potential Clinical and Research Applications