Type-specific prevalence of high-risk human papillomavirus by cervical cytology and age: Data from the health check-ups of 7,014 Korean women
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea. smkimmd@snu.ac.kr
- 2Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Korea.
- KMID: 1425658
- DOI: http://doi.org/10.5468/OGS.2013.56.2.110
Abstract
OBJECTIVE
We investigated the type-specific high-risk human papillomavirus (HPV) prevalence and distribution according to cervical cytology and age in healthy Korean women.
METHODS
A retrospective cross-sectional study was conducted with 7,014 consecutive subjects undergoing both liquid-based cervical cytology and HPV genotyping test by DNA chip for cervical cancer screening. The type-specific prevalence and distribution of individual high-risk HPV types were assessed according to cervical cytology and age groups (<30, 30-39, 40-49, 50-59, and > or =60 years old).
RESULTS
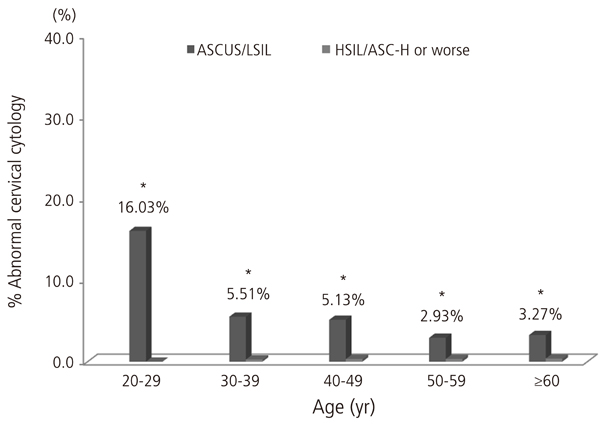
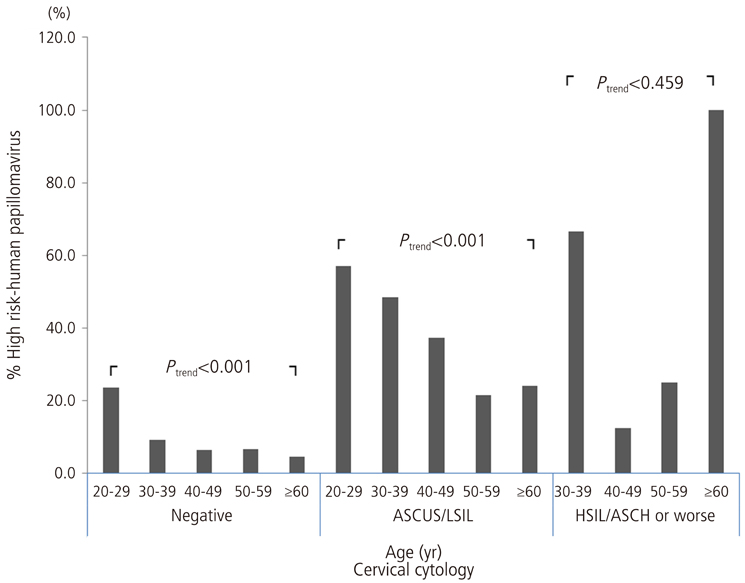
In total, the most common HPV genotype was HPV58 (23.9% of all high-risk HPV-positive subjects), followed by HPV16 (21.8%), HPV52 (16.6%), and HPV18 (11.7%). Regarding cervical cytology and age groups, the proportion of HPV56 strongly increased with the increasing severity of cervical cytology (P for trend=0.041). An age-specific decline in the overall high-risk HPV prevalence was reaffirmed, and the proportion of HPV52 declined markedly with age (P for trend=0.014).
CONCLUSION
The type-specific prevalence of high-risk HPV types significantly varies according to cervical cytology and age. It may imply that these types have different to develop into precancerous lesions in normal cervix.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Sequencing analysis of HPV-other type on an HPV DNA chip
Min-Jeong Kim, Jin Ju Kim, Sunmie Kim
Obstet Gynecol Sci. 2018;61(2):235-241. doi: 10.5468/ogs.2018.61.2.235.Proposal for cervical cancer screening in the era of HPV vaccination
Yung-Taek Ouh, Jae Kwan Lee
Obstet Gynecol Sci. 2018;61(3):298-308. doi: 10.5468/ogs.2018.61.3.298.Investigation of human papillomavirus prevalence in married women and molecular characterization and phylogenetic analysis of the virus
Ayse Erdem Yayla, Berrin Goktug Kadioglu, Ayse Aydin, Osman Aktas
Obstet Gynecol Sci. 2019;62(4):264-272. doi: 10.5468/ogs.2019.62.4.264.Prevalence of human papillomavirus genotypes and precancerous cervical lesions in a screening population in the Republic of Korea, 2014–2016
Yung-Taek Ouh, Kyung-Jin Min, Hyun Woong Cho, Moran Ki, Jin-Kyoung Oh, Sang Yop Shin, Jin Hwa Hong, Jae-Kwan Lee
J Gynecol Oncol. 2018;29(1):. doi: 10.3802/jgo.2018.29.e14.
Reference
-
1. Wright TC Jr, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med. 2003. 348:489–490.2. Wright TC Jr, Schiffman M, Solomon D, Cox JT, Garcia F, Goldie S, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004. 103:304–309.3. Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008. 26:Suppl 10. K29–K41.4. Meijer CJ, Snijders PJ, Castle PE. Clinical utility of HPV genotyping. Gynecol Oncol. 2006. 103:12–17.5. Huh W, Einstein MH, Herzog TJ, Franco EL. What is the role of HPV typing in the United States now and in the next five years in a vaccinated population? Gynecol Oncol. 2010. 117:481–485.6. Clifford G, Franceschi S, Diaz M, Munoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006. 24:Suppl 3. S3/26–S3/34.7. Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007. 121:621–632.8. Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Hansson BG, et al. HPV type-specific risks of high-grade CIN during 4 years of follow-up: a population-based prospective study. Br J Cancer. 2007. 97:129–132.9. Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005. 14:1157–1164.10. Wheeler CM, Hunt WC, Joste NE, Key CR, Quint WG, Castle PE. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. J Natl Cancer Inst. 2009. 101:475–487.11. Oh JK, Alemany L, Suh JI, Rha SH, Munoz N, Bosch FX, et al. Type-specific human papillomavirus distribution in invasive cervical cancer in Korea, 1958-2004. Asian Pac J Cancer Prev. 2010. 11:993–1000.12. Bae JH, Lee SJ, Kim CJ, Hur SY, Park YG, Lee WC, et al. Human papillomavirus (HPV) type distribution in Korean women: a meta-analysis. J Microbiol Biotechnol. 2008. 18:788–794.13. Lee HS, Kim KM, Kim SM, Choi YD, Nam JH, Park CS, et al. Human papillomavirus genotyping using HPV DNA chip analysis in Korean women. Int J Gynecol Cancer. 2007. 17:497–501.14. Monsonego J, Zerat L, Syrjanen K, Zerat JC, Smith JS, Halfon P. Prevalence of type-specific human papillomavirus infection among women in France: Implications for screening, vaccination, and a future generation of multivalent HPV vaccines. Vaccine. 2012. 30:5215–5221.15. Rhee JE, Shin MY, Kim CM, Kee HY, Chung JK, Min SK, et al. Prevalence of human papillomavirus infection and genotype distribution among high-risk Korean women for prospecting the strategy of vaccine development. Virol J. 2010. 7:201.16. Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, et al. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004. 190:468–476.17. Kim KH, Yoon MS, Na YJ, Park CS, Oh MR, Moon WC. Development and evaluation of a highly sensitive human papillomavirus genotyping DNA chip. Gynecol Oncol. 2006. 100:38–43.18. Choi YD, Han CW, Chung WJ, Jung WW, Lee JS, Nam JH, et al. Analysis of HPV-other samples by performing HPV DNA sequencing. Korean J Pathol. 2009. 43:250–253.19. Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004. 111:278–285.20. Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002. 287:2114–2119.21. Castle PE, Fetterman B, Thomas Cox J, Shaber R, Poitras N, Lorey T, et al. The age-specific relationships of abnormal cytology and human papillomavirus DNA results to the risk of cervical precancer and cancer. Obstet Gynecol. 2010. 116:76–84.22. Apgar BS, Kittendorf AL, Bettcher CM, Wong J, Kaufman AJ. Update on ASCCP consensus guidelines for abnormal cervical screening tests and cervical histology. Am Fam Physician. 2009. 80:147–155.23. Trottier H, Mahmud SM, Lindsay L, Jenkins D, Quint W, Wieting SL, et al. Persistence of an incident human papillomavirus infection and timing of cervical lesions in previously unexposed young women. Cancer Epidemiol Biomarkers Prev. 2009. 18:854–862.24. Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003. 88:63–73.25. Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003. 89:101–105.26. Choi SH, Baik KD, Lee SI. An analysis of HPV subtype in the uterine cervix and clinical usefulness of HPV DNA chip test. Korean J Obstet Gynecol. 2007. 50:465–475.27. Lee GY, Kim SM, Rim SY, Choi HS, Park CS, Nam JH. Human papillomavirus (HPV) genotyping by HPV DNA chip in cervical cancer and precancerous lesions. Int J Gynecol Cancer. 2005. 15:81–87.28. de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007. 7:453–459.29. Sargent A, Bailey A, Almonte M, Turner A, Thomson C, Peto J, et al. Prevalence of type-specific HPV infection by age and grade of cervical cytology: data from the ARTISTIC trial. Br J Cancer. 2008. 98:1704–1709.30. Kjaer SK, Breugelmans G, Munk C, Junge J, Watson M, Iftner T. Population-based prevalence, type- and age-specific distribution of HPV in women before introduction of an HPV-vaccination program in Denmark. Int J Cancer. 2008. 123:1864–1870.31. Baandrup L, Munk C, Andersen KK, Junge J, Iftner T, Kjaer SK. HPV16 is associated with younger age in women with cervical intraepithelial neoplasia grade 2 and 3. Gynecol Oncol. 2012. 124:281–285.32. Porras C, Rodriguez AC, Hildesheim A, Herrero R, Gonzalez P, Wacholder S, et al. Human papillomavirus types by age in cervical cancer precursors: predominance of human papillomavirus 16 in young women. Cancer Epidemiol Biomarkers Prev. 2009. 18:863–865.33. Lee JK, Kim MK, Song SH, Hong JH, Min KJ, Kim JH, et al. Comparison of human papillomavirus detection and typing by hybrid capture 2, linear array, DNA chip, and cycle sequencing in cervical swab samples. Int J Gynecol Cancer. 2009. 19:266–272.34. Smits HL, Bollen LJ, Tjong AHSP, Vonk J, Van Der Velden J, Ten Kate FJ, et al. Intermethod variation in detection of human papillomavirus DNA in cervical smears. J Clin Microbiol. 1995. 33:2631–2636.35. Kim CJ, Jeong JK, Park M, Park TS, Park TC, Namkoong SE, et al. HPV oligonucleotide microarray-based detection of HPV genotypes in cervical neoplastic lesions. Gynecol Oncol. 2003. 89:210–217.36. Vaucel E, Coste-Burel M, Laboisse C, Dahlab A, Lopes P. Human papillomavirus genotype distribution in cervical samples collected in routine clinical practice at the Nantes University Hospital, France. Arch Gynecol Obstet. 2011. 284:989–998.37. Mateos Lindemann ML, Sanchez Calvo JM, Chacon de Antonio J, Sanz I, Diaz E, Rubio MD, et al. Prevalence and distribution of high-risk genotypes of HPV in women with severe cervical lesions in Madrid, Spain: importance of detecting genotype 16 and other high-risk genotypes. Adv Prev Med. 2011. 2011:269468.38. Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009. 374:301–314.39. Goldhaber-Fiebert JD, Stout NK, Ortendahl J, Kuntz KM, Goldie SJ, Salomon JA. Modeling human papillomavirus and cervical cancer in the United States for analyses of screening and vaccination. Popul Health Metr. 2007. 5:11.40. Jit M, Gay N, Soldan K, Hong Choi Y, Edmunds WJ. Estimating progression rates for human papillomavirus infection from epidemiological data. Med Decis Making. 2010. 30:84–98.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence of human papillomavirus genotypes and precancerous cervical lesions in a screening population in the Republic of Korea, 2014–2016
- Human Papillomavirus Genotype Distribution Among 18,815 Women in 13 Korean Cities and Relationship With Cervical Cytology Findings
- Human Papillomavirus Prevalence and Type Distribution Among 968 Women in South Korea
- Human Papillomavirus Prevalence in Gangwon Province Using Reverse Blot Hybridization Assay
- Human Papillomavirus Prevalence and Genotype Distribution among HIV-Infected Women in Korea