Korean J Radiol.
2012 Dec;13(6):808-811. 10.3348/kjr.2012.13.6.808.
Uremic Encephalopathy with Atypical Magnetic Resonance Features on Diffusion-Weighted Images
- Affiliations
-
- 1Department of Radiology, Wonkwang University School of Medicine & Hospital, Iksan 570-711, Korea. medicalq@hanmail.net
- KMID: 1397513
- DOI: http://doi.org/10.3348/kjr.2012.13.6.808
Abstract
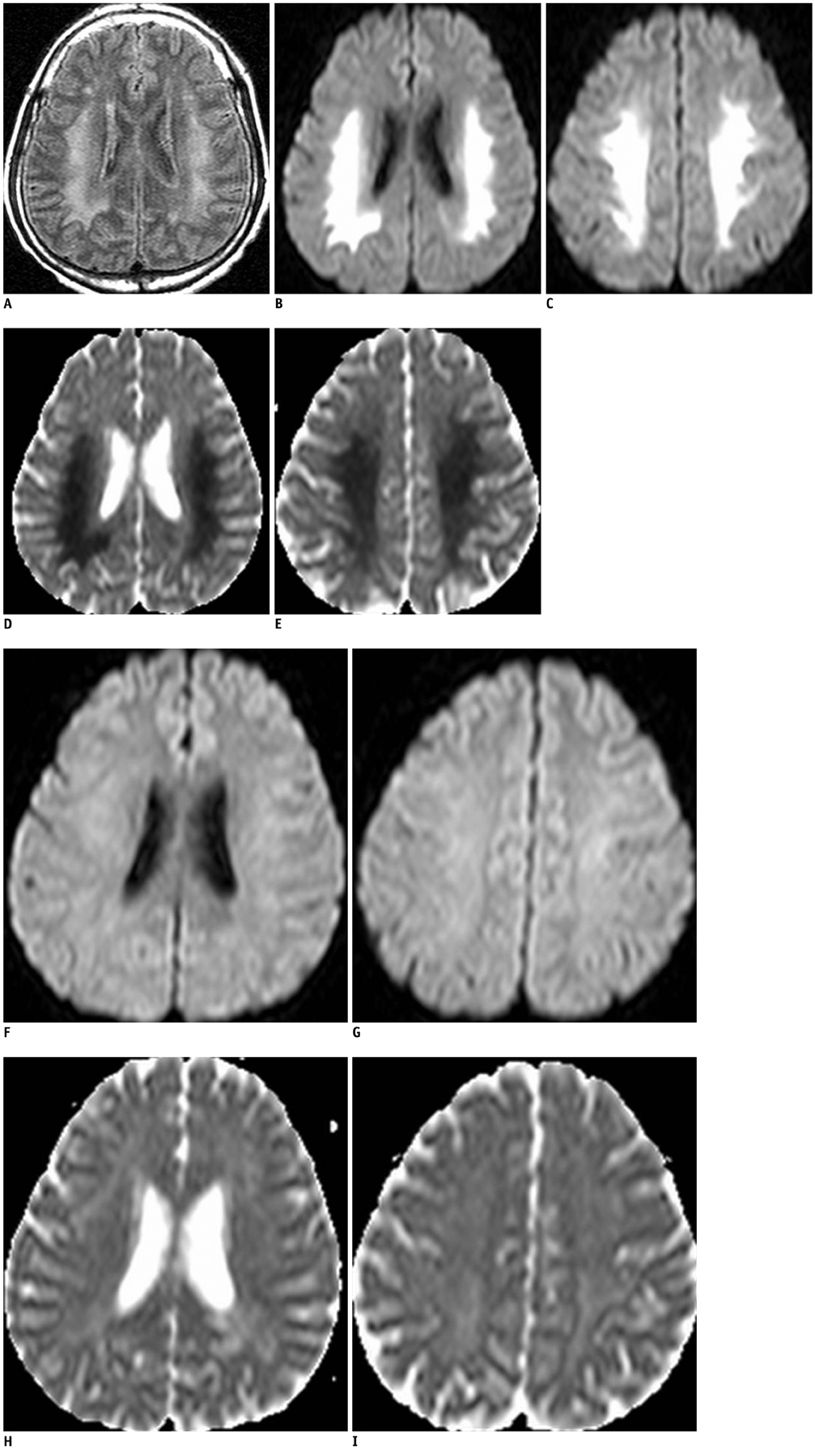
- Uremic encephalopathy is a well-known disease with typical MR findings including bilateral vasogenic or cytotoxic edema at the cerebral cortex or basal ganglia. Involvement of the basal ganglia has been very rarely reported, typically occurring in uremic-diabetic patients. We recently treated a patient who had non-diabetic uremic encephalopathy with an atypical lesion distribution involving the supratentorial white matter, without cortical or basal ganglia involvement. To the best of our knowledge, this is only the second reported case of non-diabetic uremic encephalopathy with atypical MR findings.
MeSH Terms
Figure
Reference
-
1. Wang HC, Cheng SJ. The syndrome of acute bilateral basal ganglia lesions in diabetic uremic patients. J Neurol. 2003. 250:948–955.2. Kim TK, Seo SI, Kim JH, Lee NJ, Seol HY. Diffusion-weighted magnetic resonance imaging in the syndrome of acute bilateral basal ganglia lesions in diabetic uremia. Mov Disord. 2006. 21:1267–1270.3. Raskin NH. Aminoff MJ, editor. Neurological complications of renal failure. Neurology and general medicine. 1995. New York: Churchill Livingstone;303–319.4. Raskin NH, Fishman RA. Neurologic disorders in renal failure (first of two parts). N Engl J Med. 1976. 294:143–148.5. Wang HC, Brown P, Lees AJ. Acute movement disorders with bilateral basal ganglia lesions in uremia. Mov Disord. 1998. 13:952–957.6. Prüss H, Siebert E, Masuhr F. Reversible cytotoxic brain edema and facial weakness in uremic encephalopathy. J Neurol. 2009. 256:1372–1373.7. Yoon CH, Seok JI, Lee DK, An GS. Bilateral basal ganglia and unilateral cortical involvement in a diabetic uremic patient. Clin Neurol Neurosurg. 2009. 111:477–479.8. Okada J, Yoshikawa K, Matsuo H, Kanno K, Oouchi M. Reversible MRI and CT findings in uremic encephalopathy. Neuroradiology. 1991. 33:524–526.9. Akmal M, Goldstein DA, Multani S, Massry SG. Role of uremia, brain calcium, and parathyroid hormone on changes in electroencephalogram in chronic renal failure. Am J Physiol. 1984. 246(5 Pt 2):F575–F579.10. Biasioli S, D'Andrea G, Chiaramonte S, Fabris A, Feriani M, Ronco C, et al. The role of neurotransmitters in the genesis of uremic encephalopathy. Int J Artif Organs. 1984. 7:101–106.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion Weighted Magnetic Resonance Imaging in a Patient with Acute Wernicke Encephalopathy
- Acute Hyperammonemic Encephalopathy with Features on Diffusion-Weighted Images: Report of Two Cases
- Two Cases of Hypertensive Brainstem Encephalopathy
- A Case of Metronidazole-Induced Encephalopathy: Atypical Involvement of the Brain on MRI
- Acute Acquired Metabolic Encephalopathy Based on Diffusion MRI: Note of an Error