Korean J Ophthalmol.
2012 Oct;26(5):347-354. 10.3341/kjo.2012.26.5.347.
Morphologic Changes in Acute Central Serous Chorioretinopathy Using Spectral Domain Optical Coherence Tomography
- Affiliations
-
- 1Department of Ophthalmology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea. eyekim@kuh.ac.kr
- KMID: 1397485
- DOI: http://doi.org/10.3341/kjo.2012.26.5.347
Abstract
- PURPOSE
To investigate morphologic changes of acute central serous chorioretinopathy (CSC) using spectral domain optical coherence tomography (SD-OCT) and confocal scanning laser ophthalmoscopy.
METHODS
This retrospective study included 63 eyes of 63 patients with unilateral acute CSC. All patients underwent simultaneous SD-OCT and fluorescein angiography examination using Spectralis HRA+OCT.
RESULTS
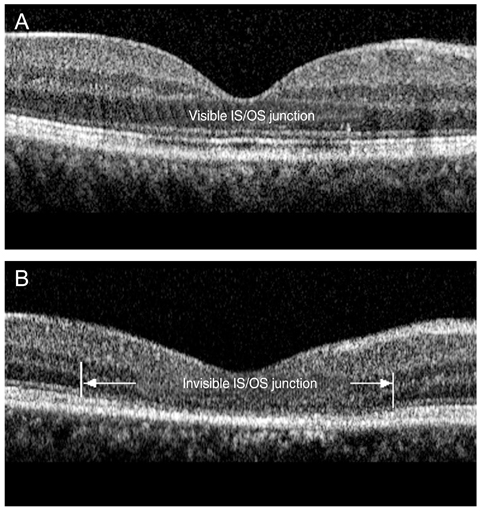
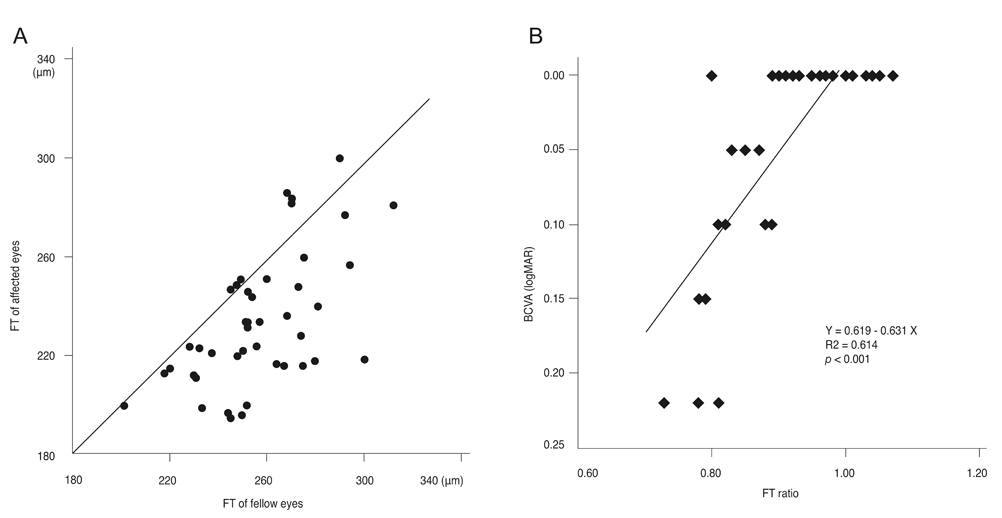
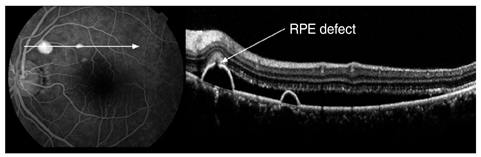
The external limiting membrane could be seen on SD-OCT, although the junction between photoreceptor inner and outer segments (IS/OS) was not detected in all eyes with retinal detachment (RD). However, IS/OS became visible after resolution of serous RD in 51 eyes (81.0%). SD-OCT images at the leakage sites showed a bump of retinal pigment epithelium (RPE) in in 47 cases (68.1%) and pigment epithelial detachment (PED) in 22 of 69 leakage sites (31.9%). In 14 of 69 leakage sites (20.3%), highly reflective areas suggesting fibrinous exudate were observed in the subretinal space. In nine leakage sites (13.0%), sagging or dipping of the posterior retinal layer was seen. Abnormal RPE changes such as RPE bump and PED were observed in 12 of 22 fellow eyes (54.5%).
CONCLUSIONS
A variety of morphologic changes could be identified on SD-OCT, and those findings may contribute more information to our understanding of the pathophysiology of CSC.
Keyword
MeSH Terms
Figure
Reference
-
1. Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967. 63:Suppl. 1–139.2. Guyer DR, Yannuzzi LA, Slakter JS, et al. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994. 112:1057–1062.3. Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996. 103:2070–2079.4. Alam S, Zawadzki RJ, Choi S, et al. Clinical application of rapid serial fourier-domain optical coherence tomography for macular imaging. Ophthalmology. 2006. 113:1425–1431.5. Chen TC, Cense B, Pierce MC, et al. Spectral domain optical coherence tomography: ultra-high speed, ultra-high resolution ophthalmic imaging. Arch Ophthalmol. 2005. 123:1715–1720.6. Hangai M, Ojima Y, Gotoh N, et al. Three-dimensional imaging of macular holes with high-speed optical coherence tomography. Ophthalmology. 2007. 114:763–773.7. Ojima Y, Hangai M, Sasahara M, et al. Three-dimensional imaging of the foveal photoreceptor layer in central serous chorioretinopathy using high-speed optical coherence tomography. Ophthalmology. 2007. 114:2197–2207.8. Wojtkowski M, Bajraszewski T, Gorczynska I, et al. Ophthalmic imaging by spectral optical coherence tomography. Am J Ophthalmol. 2004. 138:412–419.9. Wojtkowski M, Srinivasan V, Fujimoto JG, et al. Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2005. 112:1734–1746.10. Wolf-Schnurrbusch UE, Enzmann V, Brinkmann CK, Wolf S. Morphologic changes in patients with geographic atrophy assessed with a novel spectral OCT-SLO combination. Invest Ophthalmol Vis Sci. 2008. 49:3095–3099.11. Iida T, Kishi S, Hagimura N, Shimizu K. Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina. 1999. 19:508–512.12. Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina. 1994. 14:231–242.13. Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996. 121:26–34.14. Scheider A, Nasemann JE, Lund OE. Fluorescein and indocyanine green angiographies of central serous choroidopathy by scanning laser ophthalmoscopy. Am J Ophthalmol. 1993. 115:50–56.15. Hussain N, Baskar A, Ram LM, Das T. Optical coherence tomographic pattern of fluorescein angiographic leakage site in acute central serous chorioretinopathy. Clin Experiment Ophthalmol. 2006. 34:137–140.16. Iida T, Hagimura N, Sato T, Kishi S. Evaluation of central serous chorioretinopathy with optical coherence tomography. Am J Ophthalmol. 2000. 129:16–20.17. Kamppeter B, Jonas JB. Central serous chorioretinopathy imaged by optical coherence tomography. Arch Ophthalmol. 2003. 121:742–743.18. Mitarai K, Gomi F, Tano Y. Three-dimensional optical coherence tomographic findings in central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2006. 244:1415–1420.19. Fujimoto H, Gomi F, Wakabayashi T, et al. Morphologic changes in acute central serous chorioretinopathy evaluated by fourier-domain optical coherence tomography. Ophthalmology. 2008. 115:1494–1500. 1500.e1–1500.e2.20. Matsumoto H, Kishi S, Otani T, Sato T. Elongation of photoreceptor outer segment in central serous chorioretinopathy. Am J Ophthalmol. 2008. 145:162–168.21. Jalkh AE, Jabbour N, Avila MP, et al. Retinal pigment epithelium decompensation. I. Clinical features and natural course. Ophthalmology. 1984. 91:1544–1548.22. Yannuzzi LA, Shakin JL, Fisher YL, Altomonte MA. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984. 91:1554–1572.23. Matsumoto H, Sato T, Kishi S. Outer nuclear layer thickness at the fovea determines visual outcomes in resolved central serous chorioretinopathy. Am J Ophthalmol. 2009. 148:105–110.e1.24. Montero JA, Ruiz-Moreno JM. Optical coherence tomography characterisation of idiopathic central serous chorioretinopathy. Br J Ophthalmol. 2005. 89:562–564.25. Shinojima A, Hirose T, Mori R, et al. Morphologic findings in acute central serous chorioretinopathy using spectral domain-optical coherence tomography with simultaneous angiography. Retina. 2010. 30:193–202.26. Perkins SL, Kim JE, Pollack JS, Merrill PT. Clinical characteristics of central serous chorioretinopathy in women. Ophthalmology. 2002. 109:262–266.27. Eandi CM, Ober M, Iranmanesh R, et al. Acute central serous chorioretinopathy and fundus autofluorescence. Retina. 2005. 25:989–993.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spectral Domain OCT Findings of Asymptomatic Fellow Eyes in Central Serous Chorioretinopathy
- Effect of Serous Retinal Detachment on the Measurement of Axial Length in Central Serous Chorioretinopathy
- The Use fulness of OCT[Optical Coherence Tomography]for the Diagnosis of Central Serous Choriore tinopathy
- Central Serous Chorioretinopathy in a Patient with Retinal Macrovessel
- Subfoveal Choroidal Thickness in Fellow Eyes of Patients with Central Serous Chorioretinopathy