J Vet Sci.
2012 Sep;13(3):315-322. 10.4142/jvs.2012.13.3.315.
Improved assessment of frozen/thawed mouse spermatozoa using fluorescence microscopy
- Affiliations
-
- 1Cryopreservation W430, German Cancer Research Center (DKFZ), 69120 Heidelberg, Germany. j.schenkel@dkfz.de
- 2Institute of Physiology and Pathophysiology, Heidelberg University, 69120 Heidelberg, Germany.
- KMID: 1389772
- DOI: http://doi.org/10.4142/jvs.2012.13.3.315
Abstract
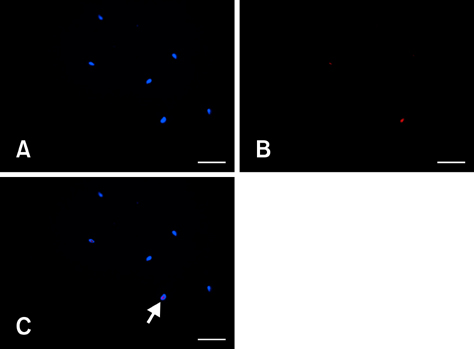
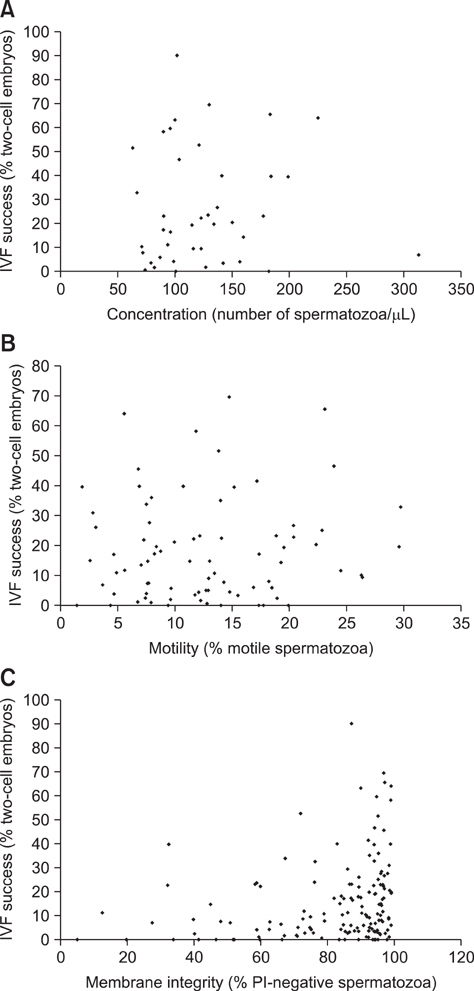
- Genetically modified (GM) animals are unique mutants with an enormous scientific potential. Cryopreservation of pre-implantation embryos or spermatozoa is a common approach for protecting these lines from being lost or to store them in a repository. A mutant line can be taken out of a breeding nucleus only if sufficient numbers of samples with an appropriate level of quality are cryopreserved. The quality of different donors within the same mouse line might be heterogeneous and the cryopreservation procedure might also be error-prone. However, only limited amounts of material are available for analysis. To improve the monitoring of frozen/thawed spermatozoa, commonly used in vitro fertilization (IVF) followed by embryo transfer were replaced with animal-free techniques. Major factors for assessing spermatozoa quality (i.e., density, viability, motility, and morphology) were evaluated by fluorescence microscopy. For this, a live/dead cell staining protocol requiring only small amounts of material was created. Membrane integrity was then examined as major parameter closely correlated with successful IVF. These complex analyses allow us to monitor frozen/thawed spermatozoa from GM mice using a relatively simple staining procedure. This approach leads to a reduction of animal experiments and contributes to the 3R principles (replacement, reduction and refinement of animal experiments).
Keyword
MeSH Terms
-
Animals
Benzimidazoles/chemistry
Cryopreservation/veterinary
Embryo Transfer/veterinary
Female
Fertilization in Vitro/veterinary
Fluorescent Dyes/chemistry
Male
Mice
Mice, Transgenic
Microscopy, Fluorescence/*methods/veterinary
Propidium/chemistry
Semen Analysis/*methods/veterinary
Semen Preservation/veterinary
Spermatozoa/*physiology
Figure
Reference
-
1. Anzar M, Kroetsch T, Buhr MM. Comparison of different methods for assessment of sperm concentration and membrane integrity with bull semen. J Androl. 2009. 30:661–668.
Article2. Barbas JP, Mascarenhas RD. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank. 2009. 10:49–62.
Article3. Bastos H, Lassalle B, Chicheportiche A, Riou L, Testart J, Allemand I, Fouchet P. Flow cytometric characterization of viable meiotic and postmeiotic cells by Hoechst 33342 in mouse spermatogenesis. Cytometry A. 2005. 65:40–49.
Article4. Dandekar PV, Glass RH. Development of mouse embryos in vitro is affected by strain and culture medium. Gamete Res. 1987. 17:279–285.
Article5. Diercks AK, Schwab A, Rittgen W, Kruspel A, Heuss E, Schenkel J. Environmental influences on the production of pre-implantation embryos. Theriogenology. 2010. 73:1238–1243.
Article6. Draper NR, Smith H. Applied Regression Analysis. 1981. 2nd ed. New York: John Wiley & Sons;43–47.7. Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J R Stat Soc. 1922. 85:87–94.
Article8. Fraser L, Parda A, Filipowicz K, Strzeżek J. Comparison of post-thaw DNA integrity of boar spermatozoa assessed with the neutral comet assay and Sperm-Sus Halomax test kit. Reprod Domest Anim. 2010. 45:e155–e160.
Article9. Gañán N, González R, Garde JJ, Martínez F, Vargas A, Gomendio M, Roldan ERS. Assessment of semen quality, sperm cryopreservation and heterologous IVF in the critically endangered Iberian lynx (Lynx pardinus). Reprod Fertil Dev. 2009. 21:848–859.
Article10. Hendricks WA, Robey KW. The sampling distribution of the coefficient of variation. Ann Math Stat. 1936. 7:129–132.
Article11. Ho Y, Wigglesworth K, Eppig JJ, Schultz RM. Preimplantation development of mouse embryos in KSOM: augmentation by amino acids and analysis of gene expression. Mol Reprod Dev. 1995. 41:232–238.
Article12. Hollander M, Wolfe DA. Nonparametric Statistical Methods. 1973. 1st ed. New York: John Wiley & Sons;191–192.13. Lezcano M, Granja C, Salazar M. The use of flow cytometry in the evaluation of cell viability of cryopreserved sperm of the marine shrimp (Litopenaeus vannamei). Cryobiology. 2004. 48:349–356.
Article14. Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: a Laboratory Manual. 2003. 3rd ed. New York: Cold Spring Harbor Laboratory Press;155–156.15. Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: a Laboratory Manual. 2003. 3rd ed. New York: Cold Spring Harbor Laboratory Press;570–572.16. Nicklas W, Baneux P, Boot R, Decelle T, Deeny AA, Fumanelli M, Illgen-Wilcke B. FELASA (Federation of European Laboratory Animal Science Associations) Working Group on Health Monitoring of Rodent and Rabbit Colonies. Federation of European Laboratory Animal Science Associations) Working Group on Health Monitoring of Rodent and Rabbit Colonies. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab Anim. 2002. 36:20–42.
Article17. Ostermeier GC, Wiles MV, Farley JS, Taft RA. Conserving, distributing and managing genetically modified mouse lines by sperm cryopreservation. PLoS One. 2008. 3:e2792.
Article18. Partyka A, Niżański W, Łukaszewicz E. Evaluation of fresh and frozen-thawed fowl semen by flow cytometry. Theriogenology. 2010. 74:1019–1027.
Article19. Petrunkina AM, Waberski D, Günzel-Apel AR, Töpfer-Petersen E. Determinants of sperm quality and fertility in domestic species. Reproduction. 2007. 134:3–17.
Article20. Russell WMS, Burch RL. The Principles of Humane Experimental Technique. 1959. London: Methuen;69–153.21. Said TM, Tellez S, Evenson DP, Del Valle AP. Assessment of sperm quality, DNA integrity and cryopreservation protocols in men diagnosed with testicular and systemic malignancies. Andrologia. 2009. 41:377–382.
Article22. Schwab A, Schenkel J. Collection, cryopreservation, storage, and revitalization of transgenic mouse embryos. CSH Protoc. 2008. 2008:pdb.prot5111.
Article23. Songsasen N, Leibo SP. Cryopreservation of mouse spermatozoa. II. Relationship between survival after cryopreservation and osmotic tolerance of spermatozoa from three strains of mice. Cryobiology. 1997. 35:255–269.24. Suzuki O, Asano T, Yamamoto Y, Takano K, Koura M. Development in vitro of preimplantation embryos from 55 mouse strains. Reprod Fertil Dev. 1996. 8:975–980.25. Sztein JM, Farley JS, Mobraaten LE. In vitro fertilization with cryopreserved inbred mouse sperm. Biol Reprod. 2000. 63:1774–1780.26. Sztein JM, Farley JS, Young AF, Mobraaten LE. Motility of cryopreserved mouse spermatozoa affected by temperature of collection and rate of thawing. Cryobiology. 1997. 35:46–52.
Article27. Tada N, Sato M, Yamanoi J, Mizorogi T, Kasai K, Ogawa S. Cryopreservation of mouse spermatozoa in the presence of raffinose and glycerol. J Reprod Fertil. 1990. 89:511–516.
Article28. Walters EM, Men H, Agca Y, Mullen SF, Critser ES, Critser JK. Osmotic tolerance of mouse spermatozoa from various genetic backgrounds: acrosome integrity, membrane integrity, and maintenance of motility. Cryobiology. 2005. 50:193–205.
Article29. Woods EJ, Benson JD, Agca Y, Critser JK. Fundamental cryobiology of reproductive cells and tissues. Cryobiology. 2004. 48:146–156.
Article30. Yamashita M, Yamagata K, Tsumura K, Nakanishi T, Baba T. Acrosome reaction of mouse epididymal sperm on oocyte zona pellucida. J Reprod Dev. 2007. 53:255–262.
Article31. Yildiz C, Fleming C, Ottaviani P, McKerlie C. Fresh and frozen-thawed sperm quality, nuclear DNA integrity, in vitro fertility, embryo development, and live-born offspring of N-ethyl-N-nitrosourea (ENU) mice. Cryobiology. 2008. 57:156–162.
Article32. Yildiz C, Ottaviani P, Law N, Ayearst R, Liu L, McKerlie C. Effects of cryopreservation on sperm quality, nuclear DNA integrity, in vitro fertilization, and in vitro embryo development in the mouse. Reproduction. 2007. 133:585–595.
Article33. Zeng HT, Tulsiani DRP. Calmodulin antagonists differentially affect capacitation-associated protein tyrosine phosphorylation of mouse sperm components. J Cell Sci. 2003. 116:1981–1989.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Fertilization Promoting Peptide on Kinematic Parameters, Capacitaion and Acrosome Reaction in Human Spermatozoa
- Efficacy and Fertilizing Ability of Frozen-thawed Testicular Spermatozoa and Spermatozoa Extracted from the Seminiferous Tubule with Intracytoplasmic Sperm Injection (ICSI)
- A successful pregnancy using completely immotile but viable frozen-thawed spermatozoa selected by laser
- Effect of Human Oviduct Epithelial Cells and Vero Cell on Early Mouse Embryonal Development In Vitro
- A Case of Pregnancy from Embryos following ICSI with Frozen-Thawed Testicular Sperms