J Vet Sci.
2012 Sep;13(3):223-227. 10.4142/jvs.2012.13.3.223.
Evidence for estrogen receptor expression during medullary bone formation and resorption in estrogen-treated male Japanese quails (Coturnix coturnix japonica)
- Affiliations
-
- 1Department of Oral Biology, Graduate School of Biomedical Sciences, Hiroshima University, Hiroshima 734-8553, Japan. hiyamas@hiroshima-u.ac.jp
- 2Department of Animal Science, Faculty of Agriculture, Niigata University, Niigata 950-2181, Japan.
- KMID: 1389760
- DOI: http://doi.org/10.4142/jvs.2012.13.3.223
Abstract
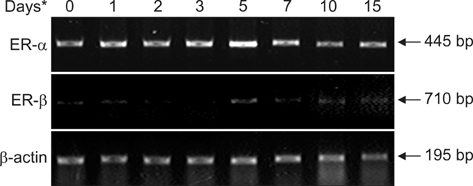
- The temporal expression of estrogen receptor (ER)-alpha and ER-beta mRNA was examined in male Japanese quails. Femurs of quails receiving 17beta-estradiol underwent RTPCR and histochemical analysis 1 to 15 days after treatment. Untreated quails were used as controls (day 0). Between days 0 and 5, cells lining the bone endosteal surface differentiated into osteoblasts, which in turn formed medullary bone. Expression of ER-alpha was already observed on day 0 and increased slightly during bone formation whereas ER-beta was hardly detected throughout this process. After osteoclasts appeared on the medullary bone surface, this type of bone disappeared from the bone marrow cavity (days 7~15). ER-alpha expression simultaneously decreased slightly and ER-beta levels remained very low. These results suggest that estrogen activity mediated by ER-alpha not only affects medullary bone formation but also bone resorption.
MeSH Terms
-
Animals
Bone Resorption/genetics
Bone and Bones/chemistry/cytology/*metabolism
Cells, Cultured
Coturnix/*metabolism
Estradiol/*pharmacology
Estrogen Receptor alpha/genetics/*metabolism
Estrogen Receptor beta/genetics/*metabolism
Gene Expression Regulation
Male
Osteoblasts/chemistry/cytology/*metabolism
Osteogenesis/genetics
RNA, Messenger/metabolism
Reverse Transcriptase Polymerase Chain Reaction
Figure
Reference
-
1. Andersson N, Islander U, Egecioglu E, Löf E, Swanson C, Movérare-Skrtic S, Sjögren K, Lindberg MK, Carlsten H, Ohlsson C. Investigation of central versus peripheral effects of estradiol in ovariectomized mice. J Endocrinol. 2005. 187:303–309.
Article2. Batra GS, Hainey L, Freemont AJ, Andrew G, Saunders PT, Hoyland JA, Braidman IP. Evidence for cell-specific changes with age in expression of oestrogen receptor (ER) α and β in bone fractures from men and women. J Pathol. 2003. 200:65–73.
Article3. Bloom W, Bloom MA, McLean FC. Calcification and ossification. Medullary bone changes in the reproductive cycle of female pigeons. Anat Rec. 1941. 81:443–475.
Article4. Bord S, Horner A, Beavan S, Compston J. Estrogen receptors α and β are differentially expressed in developing human bone. J Clin Endocrinol Metab. 2001. 86:2309–2314.
Article5. Chow JW, Lean JM, Chambers TJ. 17 beta-estradiol stimulates cancellous bone formation in female rats. Endocrinology. 1992. 130:3025–3032.
Article6. Hiyama S, Sugiyama T, Kusuhara S, Uchida T. Evidence for the expression of estrogen receptors in osteogenic cells isolated from hen medullary bone. Acta Histochem. 2009. 111:501–507.
Article7. Hoyland JA, Mee AP, Baird P, Braidman IP, Mawer EB, Freemont AJ. Demonstration of estrogen receptor mRNA in bone using in situ reverse-transcriptase polymerase chain reaction. Bone. 1997. 20:87–92.
Article8. Imamura T, Sugiyama T, Kusuhara S. Expression and localization of estrogen receptors α and β mRNA in medullary bone of laying hens. Anim Sci J. 2006. 77:223–229.
Article9. Krust A, Green S, Argos P, Kumar V, Walter P, Bornert JM, Chambon P. The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J. 1986. 5:891–897.
Article10. Kusuhara S, Schraer H. Cytology and autoradiography of estrogen-induced differentiation of avian endosteal cells. Calcif Tissue Int. 1982. 34:352–358.
Article11. Masuyama A, Ouchi Y, Sato F, Hosoi T, Nakamura T, Orimo H. Characteristics of steroid hormone receptors in cultured MC3T3-E1 osteoblastic cells and effect of steroid hormones on cell proliferation. Calcif Tissue Int. 1992. 51:376–381.
Article12. Miller SC, Bowman BM. Medullary bone osteogenesis following estrogen administration to mature male Japanese quail. Dev Biol. 1981. 87:52–63.
Article13. Mödder UIL, Riggs BL, Spelsberg TC, Fraser DG, Atkinson EJ, Arnold R, Khosla S. Dose-response of estrogen on bone versus the uterus in ovariectomized mice. Eur J Endocrinol. 2004. 151:503–510.
Article14. Notelovitz M. Estrogen therapy and osteoporosis: principles & practice. Am J Med Sci. 1997. 313:2–12.
Article15. Ohashi T, Kusuhara S. Immunoelectron microscopic detection of estrogen target cells in the bone marrow of estrogen-treated male Japanese quail. Bone Miner. 1993. 20:31–39.
Article16. Ohashi T, Kusuhara S, Ishida K. Estrogen target cells during the early stage of medullary bone osteogenesis: immunohistochemical detection of estrogen receptors in osteogenic cells of estrogen-treated male Japanese quail. Calcif Tissue Int. 1991. 49:124–127.
Article17. Oreffo ROC, Kusec V, Virdi AS, Flanagan AM, Grano M, Zambonin-Zallone A, Triffitt JT. Expression of estrogen receptor-alpha in cells of the osteoclastic lineage. Histochem Cell Biol. 1999. 111:125–133.
Article18. Oursler MJ, Osdoby P, Pyfferoen J, Riggs BL, Spelsberg TC. Avian osteoclasts as estrogen target cells. Proc Natl Acad Sci USA. 1991. 88:6613–6617.
Article19. Oursler MJ, Pederson L, Pyfferoen J, Osdoby P, Fitzpatrick L, Spelsberg TC. Estrogen modulation of avian osteoclast lysosomal gene expression. Endocrinology. 1993. 132:1373–1380.
Article20. Pederson L, Kremer M, Foged NT, Winding B, Ritchie C, Fitzpatrick LA, Oursler MJ. Evidence of a correlation of estrogen receptor level and avian osteoclast estrogen responsiveness. J Bone Miner Res. 1997. 12:742–752.
Article21. Riddle O, Rauch VM, Smith GC. Action of estrogen on plasma calcium and endosteal bone formation in parathyroidectomized pigeons. Endocrinology. 1945. 36:41–47.
Article22. Robinson JA, Harris SA, Riggs BL, Spelsberg TC. Estrogen regulation of human osteoblastic cell proliferation and differentiation. Endocrinology. 1997. 138:2919–2927.
Article23. Saintier D, Burde MA, Rey JM, Maudelonde T, de Vernejoul MC, Cohen-Solal ME. 17β-estradiol downregulates β3-integrin expression in differentiating and mature human osteoclasts. J Cell Physiol. 2004. 198:269–276.
Article24. Samuels A, Perry MJ, Goodship AE, Fraser WD, Tobias JH. Is high-dose estrogen-induced osteogenesis in the mouse mediated by an estrogen receptor? Bone. 2000. 27:41–46.
Article25. Samuels A, Perry MJ, Tobias JH. High-dose estrogen induces de novo medullary bone formation in female mice. J Bone Miner Res. 1999. 14:178–186.
Article26. Shevde NK, Bendixen AC, Dienger KM, Pike JW. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci USA. 2000. 97:7829–7834.
Article27. Sørensen MG, Henriksen K, Dziegiel MH, Tankó LB, Karsdal MA. Estrogen directly attenuates human osteoclastogenesis, but has no effect on resorption by mature osteoclasts. DNA Cell Biol. 2006. 25:475–483.
Article28. Stossi F, Barnett DH, Frasor J, Komm B, Lyttle CR, Katzenellenbogen BS. Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER) α or ERβ in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology. 2004. 145:3473–3486.
Article29. Turner RT, Colvard DS, Spelsberg TC. Estrogen inhibition of periosteal bone formation in rat long bones: down-regulation of gene expression for bone matrix proteins. Endocrinology. 1990. 127:1346–1351.
Article30. Westerlind KC, Sarkar G, Bolander ME, Turner RT. Estrogen receptor mRNA is expressed in vivo in rat calvarial periosteum. Steroids. 1995. 60:484–487.
Article31. White R, Lees JA, Needham M, Ham J, Parker M. Structural organization and expression of the mouse estrogen receptor. Mol Endocrinol. 1987. 1:735–744.
Article32. Windahl SH, Norgård M, Kuiper GGJM, Gustafsson JÅ, Andersson G. Cellular distribution of estrogen receptor β in neonatal rat bone. Bone. 2000. 26:117–121.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Idiopathic intranuclear inclusion bodies in the renal tubular epithelia of Japanese quail (Coturnix coturnix japonica)
- Bone Morphogenetic Protein-2 Desensitizes MC3T3-E1 Osteoblastic Cells to Estrogen Through Transcriptional Downregulation of Estrogen Receptor 1
- Recent Advances in the Drug Therapy of Osteoporosis
- Overexpression of Estrogen Receptor in Female Patients with Varicose Vein of Lower Extremities
- Effects of 17beta-Estradiol and Estrogen Receptor Antagonists on the Proliferation of Gastric Cancer Cell Lines