Yonsei Med J.
2006 Oct;47(5):721-728. 10.3349/ymj.2006.47.5.721.
Intracellular Antibody Fragment Against Hepatitis B Virus X Protein Does Not Inhibit Viral Replication
- Affiliations
-
- 1Department of Microbiology, Ajou University School of Medicine, Suwon, Korea. sinsun@ajou.ac.kr
- KMID: 1381254
- DOI: http://doi.org/10.3349/ymj.2006.47.5.721
Abstract
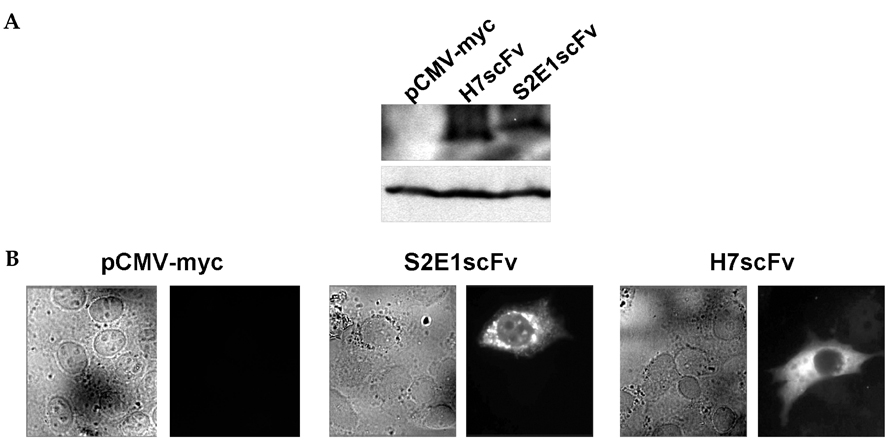
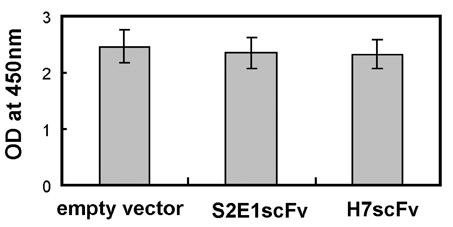
- Replication of the hepatitis B virus is suppressed by deficiency of the X protein. Although several molecules that block cellular targets of X protein reduce the production of hepatitis B virus progeny, the effect of a specific inhibitor of X protein on viral replication has not been investigated. To block X protein specifically, we adopted an intracellular expression approach using H7 single chain variable fragment (H7scFv), an antibody fragment against X protein. We previously demonstrated that cytoplasmic expression of H7scFv inhibits X protein-induced tumorigenicity and transactivation. In this study, intracellular H7scFv expression inhibits reporter gene transactivation but not viral replication determined by endogenous hepatitis B virus polymerase activity assay and real-time PCR. Our findings imply that intracellular expression of antibody fragment against X protein may not be an alternative therapeutic modality for inhibition of hepatitis B virus replication.
MeSH Terms
Figure
Reference
-
1. Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988. 61:1942–1956.2. Bouchard MJ, Schneider RJ. The enigmatic X gene of hepatitis B virus. J Virol. 2004. 78:12725–12734.3. Melegari M, Scaglioni PP, Wands JR. Cloning and characterization of a novel hepatitis B virus x binding protein that inhibits viral replication. J Virol. 1998. 72:1737–1743.4. Bouchard MJ, Wang LH, Schneider RJ. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science. 2001. 294:2376–2378.5. Xu Z, Yen TS, Wu L, Madden CR, Tan W, Slagle BL, et al. Enhancement of hepatitis B virus replication by its X protein in transgenic mice. J Virol. 2002. 76:2579–2584.6. Zhang Z, Protzer U, Hu Z, Jacob J, Liang TJ. Inhibition of cellular proteasome activities enhances hepadnavirus replication in an HBX-dependent manner. J Virol. 2004. 78:4566–4572.7. Zoulim F, Saputelli J, Seeger C. Woodchuck hepatitis virus X protein is required for viral infection in vivo. J Virol. 1994. 68:2026–2030.8. Doria M, Klein N, Lucito R, Schneider RJ. The hepatitis B virus HBx protein is a dual specificity cytoplasmic activator of Ras and nuclear activator of transcription factors. EMBO J. 1995. 14:4747–4757.9. Benn J, Su F, Doria M, Schneider RJ. Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J Virol. 1996. 70:4978–4985.10. Shirakata Y, Kawada M, Fujiki Y, Sano H, Oda M, Yaginuma K, et al. The X gene of hepatitis B virus induced growth stimulation and tumorigenic transformation of mouse NIH3T3 cells. Jpn J Cancer Res. 1989. 80:617–621.11. Kim CM, Koike K, Saito I, Miyamura T, Jay G. HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature. 1991. 351:317–320.12. Jin YH, Kwon MH, Kim K, Shin HJ, Shin JS, Cho H, et al. An intracellular antibody can suppress tumorigenicity in Hepatitis B virus X-expressing cells. Cancer Immunol Immunother. 2006. 55:569–578.13. Fu L, Cheng YC. Characterization of novel human hepatoma cell lines with stable hepatitis B virus secretion for evaluating new compounds against lamivudine- and penciclovir-resistant virus. Antimicrob Agents Chemother. 2000. 44:3402–3407.14. Chen RW, Piiparinen H, Seppanen M, Koskela P, Sarna S, Lappalainen M. Real-time PCR for detection and quantitation of hepatitis B virus DNA. J Med Virol. 2001. 65:250–256.15. Kim HY, Park GS, Kim EG, Kang SH, Shin HJ, Park S, et al. Oligomer synthesis by priming deficient polymerase in hepatitis B virus core particle. Virology. 2004. 322:22–30.16. Henkler F, Hoare J, Waseem N, Goldin RD, McGarvey MJ, Koshy R, et al. Intracellular localization of the hepatitis B virus HBx protein. J Gen Virol. 2001. 82:871–882.17. Sirma H, Weil R, Rosmorduc O, Urban S, Israel A, Kremsdorf D, et al. Cytosol is the prime compartment of hepatitis B virus X protein where it colocalizes with the proteasome. Oncogene. 1998. 16:2051–2063.18. zu Putlitz J, Skerra A, Wands JR. Intracellular expression of a cloned antibody fragment interferes with hepatitis B virus surface antigen secretion. Biochem Biophys Res Commun. 1999. 255:785–791.19. Yamamoto M, Hayashi N, Takehara T, Ueda K, Mita E, Tatsumi T, et al. Intracellular single-chain antibody against hepatitis B virus core protein inhibits the replication of hepatitis B virus in cultured cells. Hepatology. 1999. 30:300–307.20. Chen SY, Bagley J, Marasco WA. Intracellular antibodies as a new class of therapeutic molecules for gene therapy. Hum Gene Ther. 1994. 5:595–601.21. Goncalves J, Silva F, Freitas-Vieira A, Santa-Marta M, Malho R, Yang X, et al. Functional neutralization of HIV-1 Vif protein by intracellular immunization inhibits reverse transcription and viral replication. J Biol Chem. 2002. 277:32036–32045.22. Blum HE, Zhang ZS, Galun E, von Weizsacker F, Garner B, Liang TJ, et al. Hepatitis B virus X protein is not central to the viral life cycle in vitro. J Virol. 1992. 66:1223–1227.23. Reifenberg K, Nusser P, Lohler J, Spindler G, Kuhn C, von Weizsacker F, et al. Virus replication and virion export in X-deficient hepatitis B virus transgenic mice. J Gen Virol. 2002. 83(Pt 5):991–996.24. Rahmani Z, Huh KW, Lasher R, Siddiqui A. Hepatitis B virus X protein colocalizes to mitochondria with a human voltage-dependent anion channel, HVDAC3, and alters its transmembrane potential. J Virol. 2000. 74:2840–2846.25. Leupin O, Bontron S, Schaeffer C, Strubin M. Hepatitis B virus X protein stimulates viral genome replication via a DDB1-dependent pathway distinct from that leading to cell death. J Virol. 2005. 79:4238–4245.26. Huang HL, Jeng KS, Hu CP, Tsai CH, Lo SJ, Chang C. Identification and characterization of a structural protein of hepatitis B virus: a polymerase and surface fusion protein encoded by a spliced RNA. Virology. 2000. 275:398–410.27. Meyer M, Caselmann WH, Schluter V, Schreck R, Hofschneider PH, Baeuerle PA. Hepatitis B virus transactivator MHBst: activation of NF-kappa B, selective inhibition by antioxidants and integral membrane localization. EMBO J. 1992. 11:2991–3001.28. Caselmann WH, Renner M, Schluter V, Hofschneider PH, Koshy R, Meyer M. The hepatitis B virus MHBst 167 protein is a pleiotropic transactivator mediating its effect via ubiquitous cellular transcription factors. J Gen Virol. 1997. 78(Pt 6):1487–1495.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of hepatitis B virus replication by RNA interference
- Expression of Intracellular Single Chain Antibody Specific to Hepatitis B Virus X Protein
- Prevention of Viral Hepatitis and Vaccination
- The Role of Serum HBV-RNA Levels as a Marker of Intrahepatic Viral and Histologic Activity
- Impact of Nucleotide Mutations at the HNF3- and HNF4-Binding Sites in Enhancer 1 on Viral Replication in Patients with Chronic Hepatitis B Virus Infection