Korean J Radiol.
2012 Feb;13(Suppl 1):S89-S97. 10.3348/kjr.2012.13.S1.S89.
Perfusion CT in Colorectal Cancer: Comparison of Perfusion Parameters with Tumor Grade and Microvessel Density
- Affiliations
-
- 1Department of Diagnostic Radiology, Chonnam National University Medical School, Chonnam National University Hwasun Hospital, Hwasun 519-763, Korea. yjeong@chonnam.ac.kr
- 2Department of Diagnostic Radiology, Chonnam National University Medical School, Chonnam National University Hospital, Gwangju 501-757, Korea.
- 3Department of Pathology, Chonnam National University Medical School, Chonnam National University Hospital, Gwangju 501-757, Korea.
- 4Department of Surgery, Chonnam National University Medical School, Chonnam National University Hwasun Hospital, Hwasun 519-763, Korea.
- KMID: 1372873
- DOI: http://doi.org/10.3348/kjr.2012.13.S1.S89
Abstract
OBJECTIVE
The purpose of this study was to prospectively compare pre-operative computed tomography (CT) perfusion parameters with tumor grade from colorectal adenocarcinoma (CRC) and to correlate pre-operative CT perfusion parameters with microvessel density (MVD) to evaluate angiogenesis in CRC.
MATERIALS AND METHODS
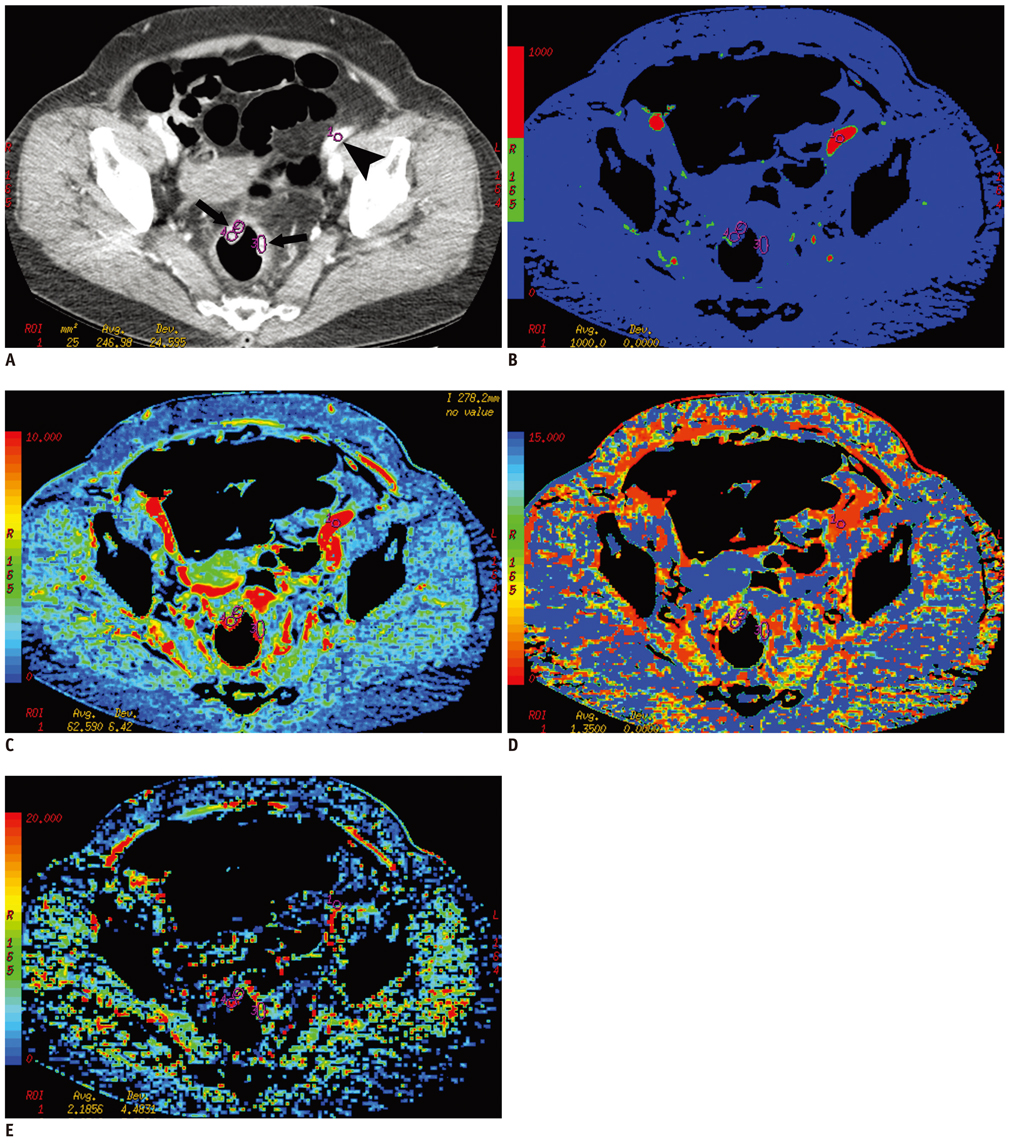
Pre-operative perfusion CTs were performed with a 64-channel multidetector row CT in 27 patients (17 women and 10 men; age range 32-82 years) who were diagnosed with CRC involving the sigmoid and rectum between August 2006 and November 2007. All patients underwent surgery without pre-operative chemotherapy or radiation therapy. Dynamic perfusion CTs were performed for 65 seconds after intravenous injection of contrast medium (100 mL, 300 mg of iodine per mL, 5 mL/sec). Before surgery, blood flow (BF), blood volume, mean transit time (MTT), and permeability-surface area product were measured in the tumor. After surgery, one gastrointestinal pathologist evaluated tumor grade and performed immunohistochemical staining using CD 34 to determine MVD in each tumor. The Kruskal-Wallis test was used to compare CT perfusion parameters with tumor grade, and Pearson's correlation analysis was used to correlate CT perfusion parameters with MVD.
RESULTS
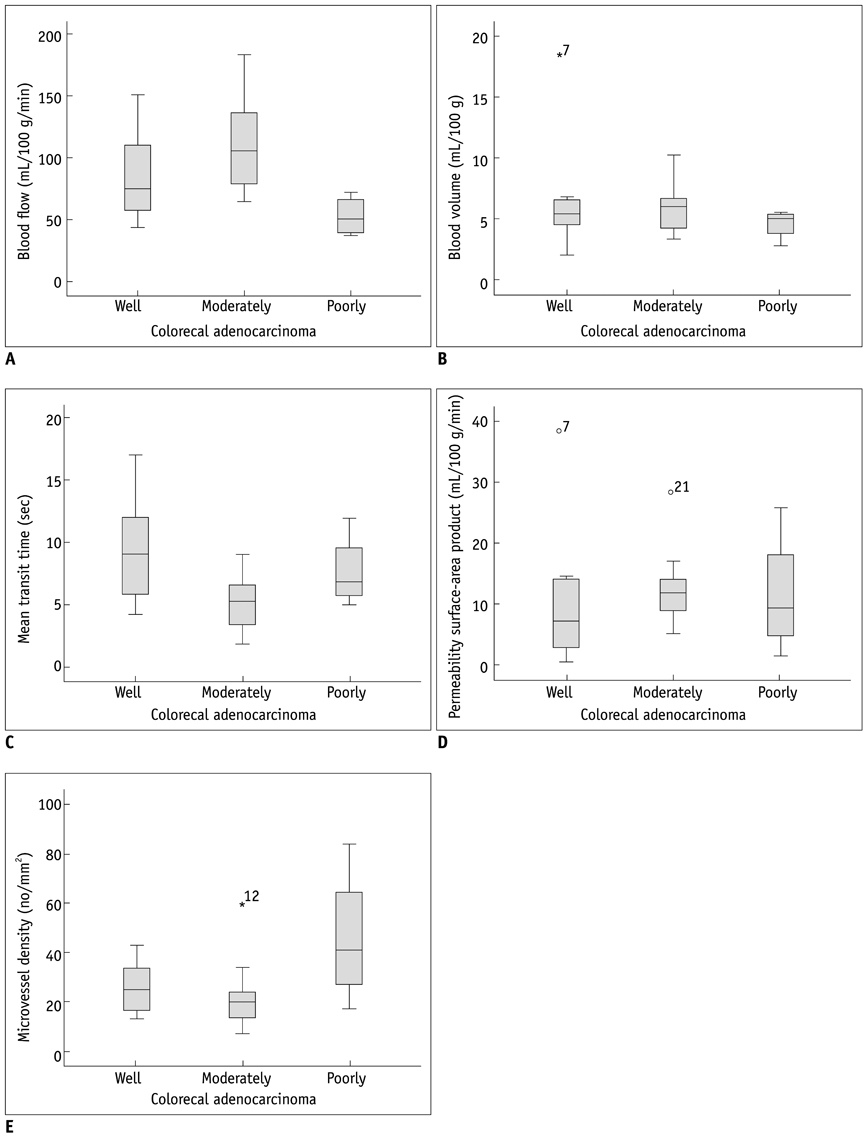
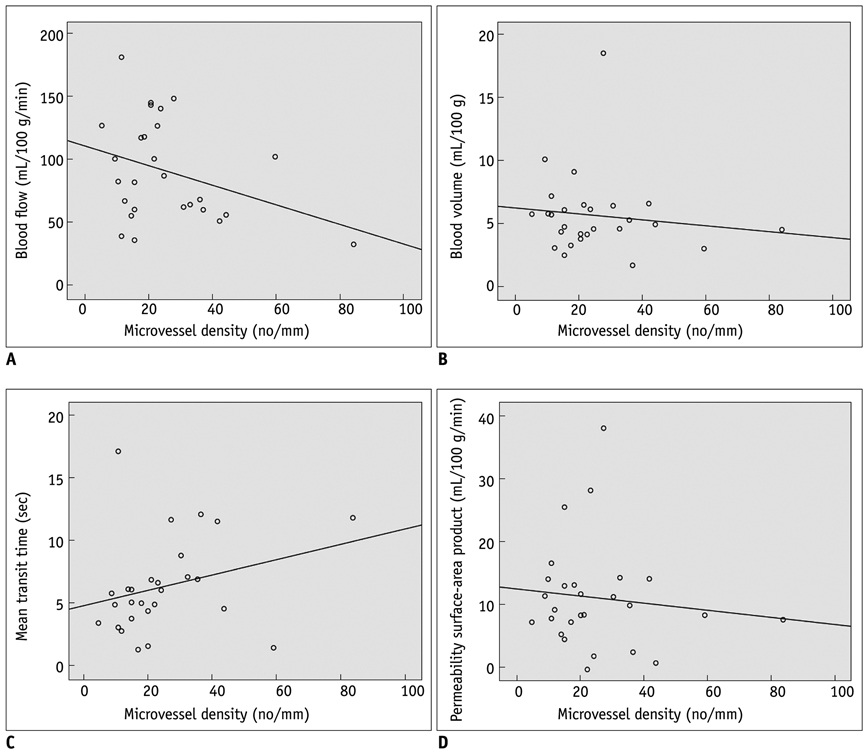
In 27 patients with CRC, tumor grading was as follows: well differentiated (n = 8); moderately differentiated (n = 15); and poorly differentiated (n = 4). BF was higher in moderately differentiated CRC than well differentiated and poorly differentiated CRCs (p = 0.14). MTT was shorter in moderately differentiated than well differentiated and poorly differentiated CRCs (p = 0.039). The MVD was greater in poorly differentiated than well differentiated and moderately differentiated CRCs (p = 0.034). There was no significant correlation between other perfusion parameters and tumor grade. There was no significant correlation between CT perfusion parameters and MVD.
CONCLUSION
BF and MTT measurement by perfusion CT is effective in predicting moderately differentiated CRCs. However, perfusion CT is limited in distinguishing well differentiated and poorly differentiated CRCs. Pre-operative perfusion CT does not reflect the MVD of CRCs.
Keyword
MeSH Terms
-
Adenocarcinoma/pathology/*radiography
Adult
Aged
Aged, 80 and over
Colorectal Neoplasms/pathology/*radiography
Contrast Media/diagnostic use
Female
Humans
Iohexol/analogs & derivatives/diagnostic use
Male
Microcirculation
Middle Aged
Neoplasm Grading
Neovascularization, Pathologic/*radiography
Prospective Studies
Statistics, Nonparametric
Tomography, X-Ray Computed/*methods
Figure
Cited by 2 articles
-
Prediction of Treatment Outcome of Chemotherapy Using Perfusion Computed Tomography in Patients with Unresectable Advanced Gastric Cancer
Dong Ho Lee, Se Hyung Kim, Sang Min Lee, Joon Koo Han
Korean J Radiol. 2019;20(4):589-598. doi: 10.3348/kjr.2018.0306.Identifying CT-Based Risk Factors Associated with Synchronous Liver Metastases in Colorectal Cancer
Cho Rong Seo, Seung Joon Choi, Hyung Sik Kim
J Korean Soc Radiol. 2017;77(5):286-297. doi: 10.3348/jksr.2017.77.5.286.
Reference
-
1. Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993. 54:594–606.2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.3. Burton S, Norman AR, Brown G, Abulafi AM, Swift RI. Predictive poor prognostic factors in colonic carcinoma. Surg Oncol. 2006. 15:71–78.4. Takebayashi Y, Aklyama S, Yamada K, Akiba S, Aikou T. Angiogenesis as an unfavorable prognostic factor in human colorectal carcinoma. Cancer. 1996. 78:226–231.5. Li C, Gardy R, Seon BK, Duff SE, Abdalla S, Renehan A, et al. Both high intratumoral microvessel density determined using CD105 antibody and elevated plasma levels of CD105 in colorectal cancer patients correlate with poor prognosis. Br J Cancer. 2003. 88:1424–1431.6. Hawighorst H, Knapstein PG, Knopp MV, Weikel W, Brix G, Zuna I, et al. Uterine cervical carcinoma: comparison of standard and pharmacokinetic analysis of time-intensity curves for assessment of tumor angiogenesis and patient survival. Cancer Res. 1998. 58:3598–3602.7. Macchiarini P, Fontanini G, Hardin MJ, Squartini F, Angeletti CA. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet. 1992. 340:145–146.8. Bossi P, Viale G, Lee AK, Alfano R, Coggi G, Bosari S. Angiogenesis in colorectal tumors: microvessel quantitation in adenomas and carcinomas with clinicopathological correlations. Cancer Res. 1995. 55:5049–5053.9. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991. 324:1–8.10. Gasparini G, Weidner N, Maluta S, Pozza F, Boracchi P, Mezzetti M, et al. Intratumoral microvessel density and p53 protein: correlation with metastasis in head-and-neck squamous-cell carcinoma. Int J Cancer. 1993. 55:739–744.11. Wakui S, Furusato M, Itoh T, Sasaki H, Akiyama A, Kinoshita I, et al. Tumour angiogenesis in prostatic carcinoma with and without bone marrow metastasis: a morphometric study. J Pathol. 1992. 168:257–262.12. Olivarez D, Ulbright T, DeRiese W, Foster R, Reister T, Einhorn L, et al. Neovascularization in clinical stage A testicular germ cell tumor: prediction of metastatic disease. Cancer Res. 1994. 54:2800–2802.13. Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, et al. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006. 94:1823–1832.14. Goh V, Halligan S, Daley F, Wellsted DM, Guenther T, Bartram CI. Colorectal tumor vascularity: quantitative assessment with multidetector CT--do tumor perfusion measurements reflect angiogenesis? Radiology. 2008. 249:510–517.15. Li ZP, Meng QF, Sun CH, Xu DS, Fan M, Yang XF, et al. Tumor angiogenesis and dynamic CT in colorectal carcinoma: radiologic-pathologic correlation. World J Gastroenterol. 2005. 11:1287–1291.16. Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995. 147:9–19.17. Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002. 38:1564–1579.18. Dugdale PE, Miles KA, Bunce I, Kelley BB, Leggett DA. CT measurement of perfusion and permeability within lymphoma masses and its ability to assess grade, activity, and chemotherapeutic response. J Comput Assist Tomogr. 1999. 23:540–547.19. Sahani DV, Holalkere NS, Mueller PR, Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue--initial experience. Radiology. 2007. 243:736–743.20. Konerding MA, Fait E, Gaumann A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Cancer. 2001. 84:1354–1362.21. Goh V, Halligan S, Gharpuray A, Wellsted D, Sundin J, Bartram CI. Quantitative assessment of colorectal cancer tumor vascular parameters by using perfusion CT: influence of tumor region of interest. Radiology. 2008. 247:726–732.22. Miles KA. Tumour angiogenesis and its relation to contrast enhancement on computed tomography: a review. Eur J Radiol. 1999. 30:198–205.23. Chen WX, Min PQ, Song B, Xiao BL, Liu Y, Ge YH. Single-level dynamic spiral CT of hepatocellular carcinoma: correlation between imaging features and density of tumor microvessels. World J Gastroenterol. 2004. 10:67–72.24. Jinzaki M, Tanimoto A, Mukai M, Ikeda E, Kobayashi S, Yuasa Y, et al. Double-phase helical CT of small renal parenchymal neoplasms: correlation with pathologic findings and tumor angiogenesis. J Comput Assist Tomogr. 2000. 24:835–842.25. Beresford MJ, Harris AL, Ah-See M, Daley F, Padhani AR, Makris A. The relationship of the neo-angiogenic marker, endoglin, with response to neoadjuvant chemotherapy in breast cancer. Br J Cancer. 2006. 95:1683–1688.26. Offersen BV, Borre M, Overgaard J. Quantification of angiogenesis as a prognostic marker in human carcinomas: a critical evaluation of histopathological methods for estimation of vascular density. Eur J Cancer. 2003. 39:881–890.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation between Tumor Angiogenesis (Microvessel Density), Metastasis and Tumor Cell Proliferation in Colorectal Carcinomas
- Evaluation of Angiogenesis of Hepatic VX2 Carcinoma and Abscess in the Rabbit Liver with Perfusion Computed Tomography
- First-Pass Perfusion Computed Tomography and Transcranial Doppler in Hydrocephalus
- Utility of Acetazolamide-challenged CT Perfusion in Patients with High-grade Carotid Stenosis
- Expression of Cyclooxygenase-2 and Tumor Microvessel Density in Colorectal Cancer