J Vet Sci.
2011 Dec;12(4):347-352. 10.4142/jvs.2011.12.4.347.
Dynamics of Moraxella bovis infection and humoral immune response to bovine herpes virus type 1 during a natural outbreak of infectious bovine keratoconjunctivitis in beef calves
- Affiliations
-
- 1Departamento de Salud Publica Veterinaria, Facultad de Ciencias Veterinarias, Universidad Nacional del Litoral, Kreder 2805, Esperanza, C.P. S3080HOF, Provincia de Santa Fe, Argentina. virginiazbrun@yahoo.com.ar
- 2Instituto Nacional de Tecnologia Agropecuaria, Estacion Experimental Agropecuaria, Marcos Juarez, Ruta 12 Km 3, M. Juarez, C.P. 2580, Provincia de Cordoba, Argentina.
- KMID: 1365018
- DOI: http://doi.org/10.4142/jvs.2011.12.4.347
Abstract
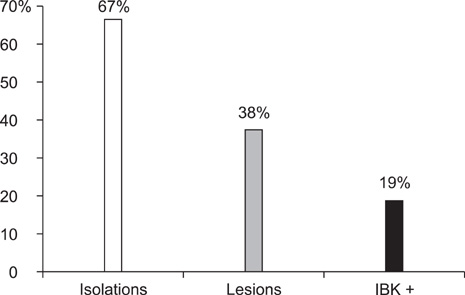
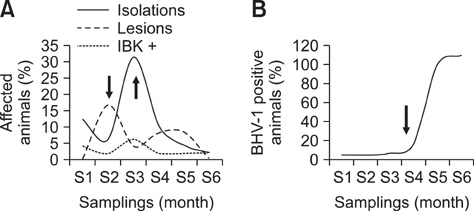
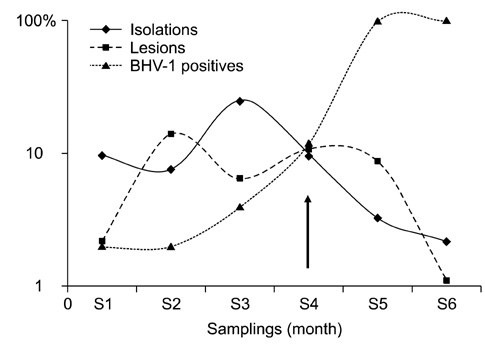
- Infectious bovine keratoconjunctivitis (IBK) is an acute disease caused by Moraxella bovis (Mb). Several factors may predispose animals to an IBK outbreak; one commonly observed is infection with bovine herpes virus type 1 (BHV-1). The aim of this study was to investigate the dynamics of BHV-1 virus infection and its relation with clinical cases of IBK in weaned calves from a beef herd with a high prevalence of lesions caused by Mb. Sampling was carried out in six stages and included conjunctival swabs for isolating Mb as well as blood samples for identifying antibodies specific for BHV-1. A score for IBK lesions after observing each eye was determined. The findings of this study showed a high prevalence of BHV-1 virus infection (100% of animals were infected at the end of the trial); 67% of animals were culture-positive for Mb, but low rates of clinical IBK (19% of calves affected) were detected at the end of the trial. These results suggest that infection with BHV-1 did not predispose these animals to IBK, and that Mb infection produced clinical and subclinical disease in the absence of BHV-1 co-infection.
MeSH Terms
Figure
Reference
-
1. Cheng TH. Frequency of pinkeye incidence in cattle in relation to face fly abundance. J Econ Entomol. 1967. 60:598–599.
Article2. George LW, Ardans A, Mihalyi J, Guerra MR. Enhancement of infectious bovine keratoconjunctivitis by modified-live infectious bovine rhinotracheitis virus vaccine. Am J Vet Res. 1988. 49:1800–1806.3. Holt JG. Bergey's Manual of Systematic Bacteriology. 1994. 9th ed. Baltimore: Williams & Wilkins;296–302.4. Kopecky KE, Pugh GW Jr, Hughes DE. Wavelength of ultraviolet radiation that enhances onset of clinical infectious bovine keratoconjunctivitis. Am J Vet Res. 1980. 41:1412–1415.5. Kopecky KE, Pugh GW Jr, Mcdonald TJ. Infectious bovine keratoconjunctivitis: Contact transmission. Am J Vet Res. 1986. 47:622–624.6. Lepper AWD, Hermans IR. Characterisation and quantitation of pilus antigens of Moraxella bovis by ELISA. Aust Vet J. 1986. 63:401–405.
Article7. Pidone CL, Galosi CM, Etcheverrigaray ME. Bovinos herpesvirus 1 y 5. Analecta Vet J. 1999. 19:40–50.8. Piscitelli HG, Zielinski GC. Evaluación de una estrategia de control de la Queratoconjuntivitis Infecciosa Bovina. Vet Arg. 1997. 14:179–186.9. Piscitelli HG, Zielinski GC. Encuesta epidemiológica sobre la queratoconjuntivitis infecciosa bovina en la provincia de Córdoba. Therios. 1996. 25:235–243.10. Prieto CI, Aguilar OM, Yantorno OM. Analyses of lipopolysaccharides, outer membrane proteins and DNA fingerprints reveal intraspecies diversity in Moraxella bovis isolated in Argentina. Vet Microbiol. 1999. 70:213–223.
Article11. Pugh GW Jr, Hughes DE, Packer RA. Bovine infectious keratoconjunctivitis: Interactions of Moraxella bovis and infectious bovine rhinotracheitis virus. Am J Vet Res. 1970. 31:653–662.12. Pugh GW Jr, Hughes DE, McDonald TJ. The isolation and characterization of Moraxella bovis. Am J Vet Res. 1966. 27:957–962.13. Pugh GW Jr, McDonald TJ. Identification of bovine carriers of Moraxella bovis by comparative cultural examinations of ocular and nasal secretions. Am J Vet Res. 1986. 47:2343–2345.14. Thielscher HH, Huth FW. IBR/IPV: Impfen oder Tilgen? Landbauforschung Völkenrode. 1986. 36:121–126.15. Wyler RM, Engels M, Schwyzer M. Wittmann G, editor. Infectious bovine rhinotracheitis/vulvo-vaginitis. Herpesvirus Diseases of Cattle, Horses, and Pigs. 1989. Boston: Kluwer Academic;1–72.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preparation and efficacy of freeze-dried inactivated vaccine against bovine viral diarrhea virus genotypes 1 and 2, bovine herpes virus type 1.1, bovine parainfluenza-3 virus, and bovine respiratory syncytial virus
- Induction of humoral responses to BHV-1 glycoprotein D expressed by HSV-1 amplicon vectors
- Infectivity of bovine leukemia virus to Korean native goats I. antibody responses and syncytium assay for Korean native goats experimentally infected with bovine leukemia virus
- Prophylactic and Therapeutic Modulation of Innate and Adaptive Immunity Against Mucosal Infection of Herpes Simplex Virus
- Investigation of bovine tuberculosis outbreaks by using a trace-back system and molecular typing in Korean Hanwoo beef cattle