J Vet Sci.
2012 Mar;13(1):33-38. 10.4142/jvs.2012.13.1.33.
The effect of conglutinin on production of reactive oxygen species in bovine granulocytes
- Affiliations
-
- 1Institute of Biological Bases of Animal Diseases, Sub-Department of Veterinary Prevention, Faculty of Veterinary Medicine, University of Life Sciences in Lublin. Akademicka 12, 20-033 Lublin, Poland. marta.dec@up.lublin.pl
- 2Department of Biotechnology, Human Nutrition and Science of Food Commodities, Faculty of Food Science and Biotechnology, University of Life Sciences in Lublin. Skromna 8, 20-704 Lublin, Poland.
- KMID: 1365000
- DOI: http://doi.org/10.4142/jvs.2012.13.1.33
Abstract
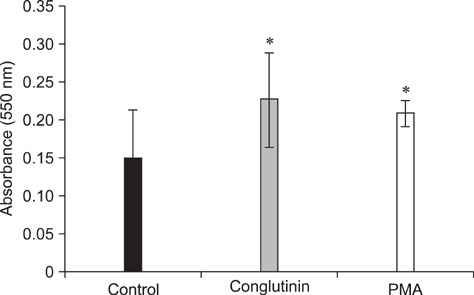
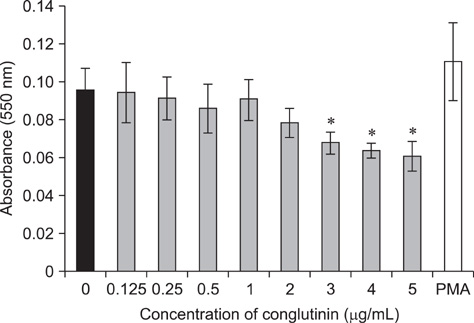
- Conglutinin is a high molecular-weight lectin originally detected in bovine serum. It belongs to the family of collectins that bind sugar residues in a Ca(2+)-dependent manner and are effector molecules in innate immunity. Conglutinin appears to play an important role in immune defense mechanisms, showing antiviral and antibacterial activities when tested in vivo and in vitro. The present study evaluated the effect of conglutinin on the respiratory bursts in bovine peripheral phagocytes. Using nitroblue tetrazolium and hydrogen peroxide assays, we showed that sugar ligand-bound conglutinin stimulated the production of superoxide and H2O2 in granulocytes whereas the non-sugar-bound form of conglutinin inhibited these processes. These results indicate that both forms of conglutinin are able to interact with surface leukocyte receptors but have opposite effects on phagocytic activity. Our findings suggest that conglutinin bound to sugar residues on microbial surfaces can induce oxygen burst in phagocytes, and thereby mediates the elimination of pathogens and prevents the spread of infection.
Keyword
MeSH Terms
-
Animals
Cattle/*immunology
Collectins/*pharmacology
Enzyme-Linked Immunosorbent Assay/veterinary
Female
Granulocytes/*drug effects/immunology
Hydrogen Peroxide/immunology
Immunity, Innate/drug effects/immunology
Phagocytosis/immunology
Reactive Oxygen Species/*immunology
Respiratory Burst/*drug effects/immunology
Serum Globulins/*pharmacology
Statistics, Nonparametric
Superoxides/immunology
Figure
Reference
-
1. Andersen O, Friis P, Holm Nielsen E, Vilsgaard K, Leslie RGQ, Svehag SE. Purification, subunit characterization and ultrastructure of three soluble bovine lectins: conglutinin, mannose-binding protein and the pentraxin serum amyloid P-component. Scand J Immunol. 1992. 36:131–141.
Article2. Babior BM. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984. 64:959–966.
Article3. Dec M, Wernicki A. Conglutinin, CL-43 and CL-46--three bovine collectins. Pol J Vet Sci. 2006. 9:265–275.4. Eggleton P, Lieu TS, Zappi EG, Sastry K, Coburn J, Zaner KS, Sontheimer RD, Capra JD, Ghebrehiwet B, Tauber AI. Calreticulin is released from activated neutrophils and binds to C1q and mannan-binding protein. Clin Immunol Immunopathol. 1994. 72:405–409.
Article5. Friis P, Svehag SE, Andersen O, Gahrn-Hansen B, Leslie RGQ. Conglutinin exhibits a complement-dependent enhancement of the respiratory burst of phagocytes stimulated by E. coli. Immunology. 1991. 74:680–684.6. Friis-Christiansen P, Thiel S, Svehag SE, Dessau R, Svendsen P, Andersen O, Laursen SB, Jensenius JC. In vivo and in vitro antibacterial activity of conglutinin, a mammalian plasma lectin. Scand J Immunol. 1990. 31:453–460.
Article7. Goodman EB, Tenner AJ. Signal transduction mechanisms of C1q-mediated superoxide production. Evidence for the involvement of temporally distinct staurosporine-insensitive and sensitive pathways. J Immunol. 1992. 148:3920–3928.8. Ingram DG, Mitchell WR. Conglutinin level in dairy cattle: changes associated with disease. Am J Vet Res. 1971. 32:875–878.9. Krogh-Meibom T, Ingvartsen KL, Tornoe I, Palaniyar N, Willis AC, Holmskov U. A simple two-step purification procedure for the iC3b binding collectin conglutinin. J Immunol Methods. 2010. 31:204–208.
Article10. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970. 227:680–685.
Article11. Laursen SB, Thiel S, Teisner B, Holmskov U, Wang Y, Sim RB, Jensenius JC. Bovine conglutinin binds to an oligosaccharide determinant presented by iC3b, but not by C3, C3b or C3c. Immunology. 1994. 81:648–654.12. Loveless RW, Feizi T, Childs RA, Mizuochi T, Stoll MS, Oldroyd RG, Lachmann PJ. Bovine serum conglutinin is a lectin which binds non-reducing terminal N-acetylglucosamine, mannose and fucose residues. Biochem J. 1989. 258:109–113.
Article13. Madan T, Eggleton P, Kishore U, Strong P, Aggrawal SS, Sarma PU, Reid KBM. Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect Immun. 1997. 65:3171–3179.
Article14. Mehrzad J, Dosogne H, Vangroenweghe F, Burvenich C. A comparative study of bovine blood and milk neutrophil functions with luminol-dependent chemiluminescence. Luminescence. 2001. 16:343–356.
Article15. Muñoz M, Cedeño R, Rodríguez J, Van der Knaap WPW, Mialhe E, Bachère E. Measurement of reactive oxygen intermediate production in haemocytes of the penaeid shrimp, Penaeus vannamei. Aquaculture. 2000. 191:89–107.
Article16. Pick E. Microassays for superoxide and hydrogen peroxide production and nitroblue tetrazolium reduction using an enzyme immunoassay microplate reader. Methods Enzymol. 1986. 132:407–421.17. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979. 76:4350–4354.
Article18. Uemura K, Yamamoto H, Nakagawa T, Nakamura K, Kawasaki N, Oka S, Ma BY, Kawasaki T. Superoxide production from human polymorphonuclear leukocytes by human mannan-binding protein (MBP). Glycoconj J. 2004. 21:79–84.
Article19. Urban-Chmiel R, Wernicki A, Puchalski A, Dec M. In vitro effect of α-tocopherol and ascorbic acid supplementation on immunological indicators in bovine leukocytes following transportation. Acta Vet Brno. 2009. 78:589–594.
Article20. van de Wetering JK, van Golde LMG, Batenburg JJ. Collectins: players of the innate immune system. Eur J Biochem. 2004. 271:1229–1249.21. van Gestelen P, Asard H, Caubergs RJ. Solubilization and Separation of a Plant Plasma Membrane NADPH-O2- Synthase from Other NAD(P)H Oxidoreductases. Plant Physiol. 1997. 115:543–550.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of High Glucose on the Production of Reactive Oxygen Species in Trabecular Meshwork Cells
- Do Reactive Oxygen Species Cause Aging?
- Effect of Allopurinol on Methylmercuric Chloride in Cultured O1igodendrocytes
- Effect of High Glucose on the Production of Reactive Oxygen Species in R28 Cells
- Protective Effect of Thiore doxin on Bovine Corneal Endothelial Cells Damaged by Oxidative Stresses