J Vet Sci.
2012 Mar;13(1):23-31. 10.4142/jvs.2012.13.1.23.
Immunomodulatory effects of human amniotic membrane-derived mesenchymal stem cells
- Affiliations
-
- 1Department of Microbiology, College of Veterinary Medicine, and BK 21 Program for Veterinary Science, Seoul National University, Seoul 151-742, Korea. koohj@snu.ac.kr, yhp@snu.ac.kr
- 2Department of Biochemistry, College of Veterinary Medicine, and BK 21 Program for Veterinary Science, Seoul National University, Seoul 151-742, Korea.
- 3Samsung Advanced Institute of Technology, Samsung Electronics, Yongin 446-712, Korea.
- 4Research Center, RNL Bio, Seoul 153-768, Korea.
- KMID: 1364999
- DOI: http://doi.org/10.4142/jvs.2012.13.1.23
Abstract
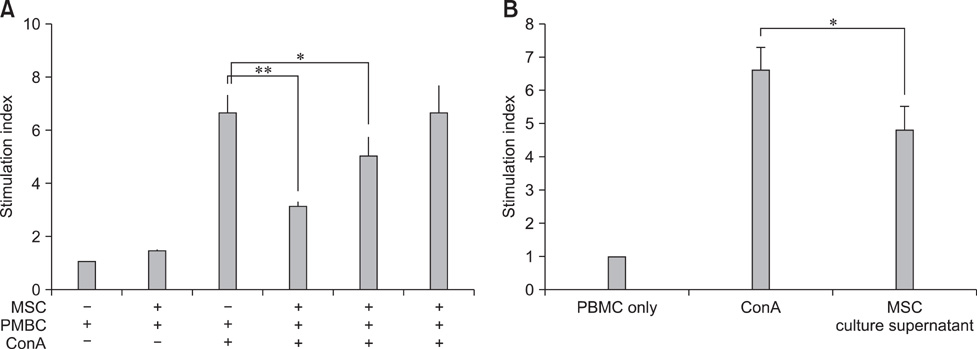
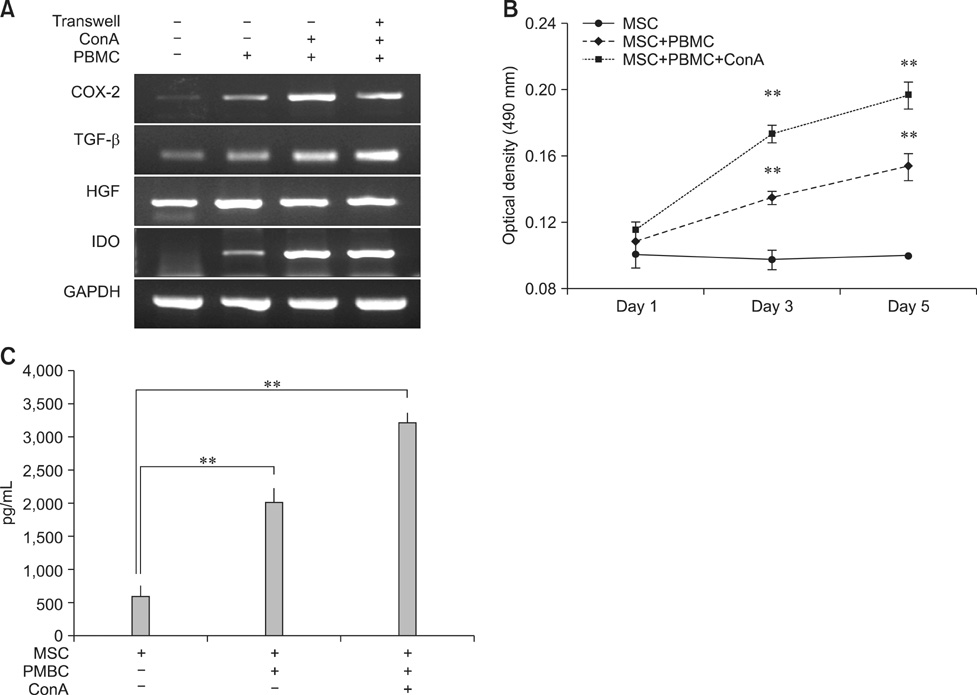
- Human amniotic membrane-derived mesenchymal stem cells (hAM-MSCs) are capable of differentiating into several lineages and possess immunomodulatory properties. In this study, we investigated the soluble factor-mediated immunomodulatory effects of hAM-MSCs. Mitogen-induced peripheral blood mononuclear cell (PBMC) proliferation was suppressed by hAM-MSCs in a dose-dependent manner as well as hAM-MSC culture supernatant. Moreover, interferon-gamma and interleukin (IL)-17 production significantly decreased from PBMC, whereas IL-10 from PBMCs and transforming growth factor beta (TGF-beta) production from hAM-MSCs significantly increased in co-cultures of hAM-MSCs and PBMCs. Production of several MSC factors, including hepatocyte growth factor (HGF), TGF-beta, prostaglandin E2 (PGE2), and indoleamine 2, 3 dioxygenase (IDO), increased significantly in hAM-MSCs co-cultured with PBMCs. These results indicate that the immunomodulatory effects of hAM-MSCs may be associated with soluble factors (TGF-beta, HGF, PGE2, and IDO), suggesting that hAM-MSCs may have potential clinical use in regenerative medicine.
MeSH Terms
-
Amnion/cytology/*immunology
Cell Differentiation/immunology
Coculture Techniques
Dinoprostone/genetics/immunology
Female
Hepatocyte Growth Factor/genetics/immunology
Humans
Immunologic Factors/*immunology
Immunophenotyping
Indoleamine-Pyrrole 2,3,-Dioxygenase/genetics/immunology
Interferon-gamma/immunology
Interleukin-10/analysis/immunology
Interleukin-17/analysis/immunology
Leukocytes, Mononuclear/cytology/immunology
Mesenchymal Stem Cells/cytology/*immunology
Pregnancy
RNA, Messenger/chemistry/genetics
Regenerative Medicine/methods
Reverse Transcriptase Polymerase Chain Reaction
Transforming Growth Factor beta/genetics/immunology
Figure
Reference
-
1. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005. 105:1815–1822.
Article2. Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002. 30:42–48.
Article3. Batten P, Sarathchandra P, Antoniw JW, Tay SS, Lowdell MW, Taylor PM, Yacoub MH. Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: relevance to tissue engineering human heart valves. Tissue Eng. 2006. 12:2263–2273.
Article4. Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, Yen BL. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006. 24:2466–2477.
Article5. Chen K, Wang D, Du WT, Han ZB, Ren H, Chi Y, Yang SG, Zhu D, Bayard F, Han ZC. Human umbilical cord mesenchymal stem cells hUC-MSCs exert immunosuppressive activities through a PGE2-dependent mechanism. Clin Immunol. 2010. 135:448–458.
Article6. Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006. 107:367–372.
Article7. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002. 99:3838–3843.
Article8. English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+ CD25High forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009. 156:149–160.
Article9. Hwang JH, Shim SS, Seok OS, Lee HY, Woo SK, Kim BH, Song HR, Lee JK, Park YK. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood, and bone marrow. J Korean Med Sci. 2009. 24:547–554.
Article10. Kang JW, Kang KS, Koo HC, Park JR, Choi EW, Park YH. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008. 17:681–693.
Article11. Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005. 12:47–57.
Article12. Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006. 24:386–398.
Article13. Le Blanc K, Rasmusson I, Götherström C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C, Ringdén O. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004. 60:307–315.
Article14. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004. 363:1439–1441.
Article15. Li C, Zhang W, Jiang X, Mao N. Human-placenta-derived mesenchymal stem cells inhibit proliferation and function of allogeneic immune cells. Cell Tissue Res. 2007. 330:437–446.
Article16. Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004. 4:762–774.
Article17. Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, Mathieu N, Sensebé L, Zhang Y, Gorin NC, Thierry D, Fouillard L. Identification of IL-10 and TGF-β transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2007. 13:217–226.
Article18. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article19. Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005. 19:1597–1604.
Article20. Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005. 129:118–129.
Article21. Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005. 305:33–41.
Article22. Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010. 184:2321–2328.
Article23. Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006. 24:74–85.
Article24. Tipnis S, Viswanathan C, Majumdar AS. Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunol Cell Biol. 2010. 88:795–806.
Article25. Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003. 75:389–397.
Article26. Yañez R, Oviedo A, Aldea M, Bueren JA, Lamana ML. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res. 2010. 316:3109–3123.
Article27. Yang SH, Park MJ, Yoon IH, Kim SY, Hong SH, Shin JY, Nam HY, Kim YH, Kim B, Park CG. Soluble mediators from mesenchymal stem cells suppress T cell proliferation by inducing IL-10. Exp Mol Med. 2009. 41:315–324.
Article28. Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005. 106:1755–1761.
Article29. Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RCH. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004. 13:263–271.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Aspects of Mesenchymal Stem Cell-Based Cell Therapy with a Focus on Human Amniotic Epithelial Cells in Multiple Sclerosis: A Mechanistic Review
- Comparison with human amniotic membrane- and adipose tissue-derived mesenchymal stem cells
- Immunomodulatory Effects of Placenta-derived Mesenchymal Stem Cells on T Cells by Regulation of FoxP3 Expression
- Progenitor Cells in Healing after Pterygium Excision
- Isolation and characterization of equine amniotic membrane-derived mesenchymal stem cells