J Vet Sci.
2012 Mar;13(1):1-6. 10.4142/jvs.2012.13.1.1.
Fast neutron irradiation deteriorates hippocampus-related memory ability in adult mice
- Affiliations
-
- 1College of Veterinary Medicine and Animal Medical Institute, Chonnam National University, Gwangju 500-757, Korea. moonc@chonnam.ac.kr
- 2Research Center, Dongnam Institute of Radiological & Medical Science, Busan 619-753, Korea.
- 3College of Veterinary Medicine and Research Institute for Subtropical Agriculture and Biotechnology, Jeju National University, Jeju 690-756, Korea.
- KMID: 1364996
- DOI: http://doi.org/10.4142/jvs.2012.13.1.1
Abstract
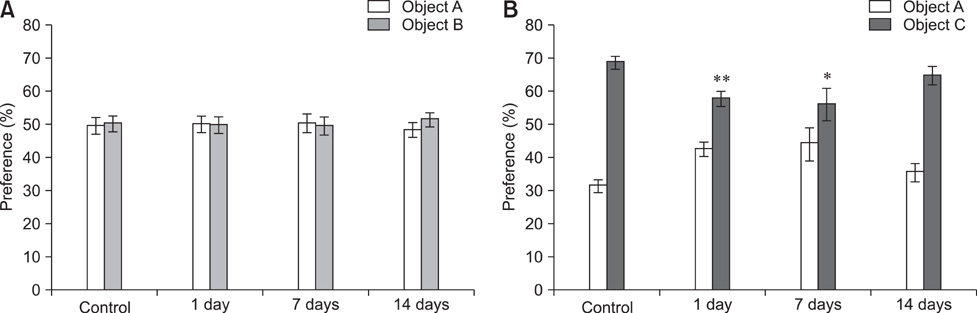
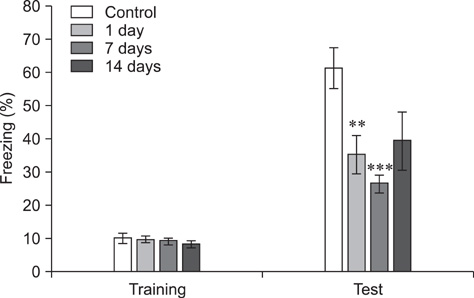
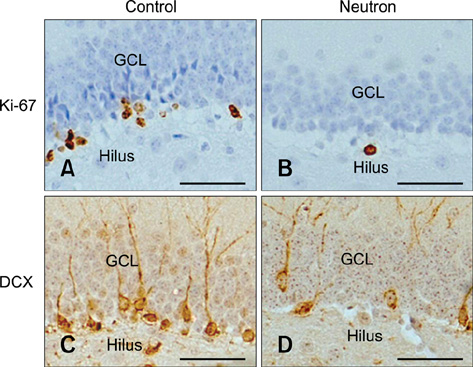
- Object recognition memory and contextual fear conditioning task performance in adult C57BL/6 mice exposed to cranial fast neutron irradiation (0.8 Gy) were examined to evaluate hippocampus-related behavioral dysfunction following acute exposure to relatively low doses of fast neutrons. In addition, hippocampal neurogenesis changes in adult murine brain after cranial irradiation were analyzed using the neurogenesis immunohistochemical markers Ki-67 and doublecortin (DCX). In the object recognition memory test and contextual fear conditioning, mice trained 1 and 7 days after irradiation displayed significant memory deficits compared to the sham-irradiated controls. The number of Ki-67- and DCX-positive cells decreased significantly 24 h post-irradiation. These results indicate that acute exposure of the adult mouse brain to a relatively low dose of fast neutrons interrupts hippocampal functions, including learning and memory, possibly by inhibiting neurogenesis.
Keyword
MeSH Terms
-
Animals
Cranial Irradiation
*Fast Neutrons
Hippocampus/metabolism/physiology/*radiation effects
Immunohistochemistry
Ki-67 Antigen/metabolism
Male
Memory/physiology/*radiation effects
Mice
Mice, Inbred C57BL
Microtubule-Associated Proteins/metabolism
Neurogenesis/physiology/*radiation effects
Neuropeptides/metabolism
Figure
Reference
-
1. Dudkin VE, Potapov YuV, Akopova AB, Melkumyan LV, Benton EV, Frank AL. Differential neutron energy spectra measured on spacecraft in low earth orbit. Int J Rad Appl Instrum D. 1990. 17:87–91.
Article2. Dyer CS, Truscott PR, Evans H, Sims AJ, Hammond N, Comber C. Secondary radiation environments in heavy space vehicles and instruments. Adv Space Res. 1996. 17:53–58.
Article3. Eguchi-Kasai K, Murakami M, Itsukaichi H, Fukutsu K, Kanai T, Furusawa Y, Sato K, Ohara H, Yatagai F. The role of DNA repair on cell killing by charged particles. Adv Space Res. 1996. 18:109–118.
Article4. Grahn D. Genetic risks associated with radiation exposures during space flight. Adv Space Res. 1983. 3:161–170.
Article5. Keith JE, Badhwar GD, Lindstrom DJ. Neutron spectrum and dose-equivalent in shuttle flights during solar maximum. Int J Rad Appl Instrum. 1992. 20:41–47.
Article6. Kim JS, Jung J, Lee HJ, Kim JC, Wang H, Kim SH, Shin T, Moon C. Differences in immunoreactivities of Ki-67 and doublecortin in the adult hippocampus in three strains of mice. Acta Histochem. 2009. 111:150–156.
Article7. Kim JS, Lee HJ, Kim JC, Kang SS, Bae CS, Shin T, Jin JK, Kim SH, Wang H, Moon C. Transient impairment of hippocampus-dependent learning and memory in relatively low-dose of acute radiation syndrome is associated with inhibition of hippocampal neurogenesis. J Radiat Res (Tokyo). 2008. 49:517–526.
Article8. Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004. 162:39–47.
Article9. Rola R, Otsuka S, Obenaus A, Nelson GA, Limoli CL, VandenBerg SR, Fike JR. Indicators of hippocampal neurogenesis are altered by 56Fe-particle irradiation in a dose-dependent manner. Radiat Res. 2004. 162:442–446.
Article10. Seo HS, Yang M, Song MS, Kim JS, Kim SH, Kim JC, Kim H, Shin T, Wang H, Moon C. Toluene inhibits hippocampal neurogenesis in adult mice. Pharmacol Biochem Behav. 2010. 94:588–594.
Article11. Shukitt-Hale B, Casadesus G, Cantuti-Castelvetri I, Rabin BM, Joseph JA. Cognitive deficits induced by 56Fe radiation exposure. Adv Space Res. 2003. 31:119–126.12. Shukitt-Hale B, Casadesus G, McEwen JJ, Rabin BM, Joseph JA. Spatial learning and memory deficits induced by exposure to iron-56-particle radiation. Radiat Res. 2000. 154:28–33.
Article13. Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005. 130:843–852.
Article14. Tada E, Parent JM, Lowenstein DH, Fike JR. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000. 99:33–41.
Article15. Yang M, Kim JS, Song MS, Kim JC, Shin T, Lee SS, Kim SH, Moon C. Dose-response and relative biological effectiveness of fast neutrons: induction of apoptosis and inhibition of neurogenesis in the hippocampus of adult mice. Int J Radiat Biol. 2010. 86:476–485.
Article16. Yang M, Kim JS, Kim J, Kim SH, Kim JC, Kim J, Wang H, Shin T, Moon C. Neurotoxicity of methotrexate to hippocampal cells in vivo and in vitro. Biochem Pharmacol. 2011. 82:72–80.
Article17. Yang M, Kim JS, Song MS, Kim SH, Kang SS, Bae CS, Kim JC, Wang H, Shin T, Moon C. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem. 2010. 93:487–494.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Combined Effect of Fast Neutron and hyperthermia according to the Sequence and Interval in MKN-45 Cells
- The RBE of Fractionated Fast Neutron on Walker 256 Carcinosarcoma with KCCH-Cyclotron
- Visuosocial Preference Memory, but Not Avoidance Memory, Requires PLCγ1 in the CA2 Hippocampus
- Correlation between Alteration of Sharp-wave Ripple Coupled Cortical Oscillation and Long-term Memory Deficit in Alzheimer Disease Model Mice
- Different Expressions of HIF-1alpha, Bcl-2 and Baxin DU145 Prostate Cancer Cells Transplanted in Nude Mouse between X-Ray and Neutron Irradiation