Korean J Ophthalmol.
2012 Apr;26(2):139-142. 10.3341/kjo.2012.26.2.139.
Intravitreal Bevacizumab for Treatment of Refractory Central Serous Choroidoretinopathy
- Affiliations
-
- 1Ophthalmic Research Center, Department of Ophthalmology, Imam Hossein Medical Center, Shahid Beheshti University (MC), Tehran, Iran. entmort@hotmail.com
- 2Ophthalmic Research Center, Department of Ophthalmology, Labbafinejad Medical Center, Shahid Beheshti University (MC), Tehran, Iran.
- KMID: 1364869
- DOI: http://doi.org/10.3341/kjo.2012.26.2.139
Abstract
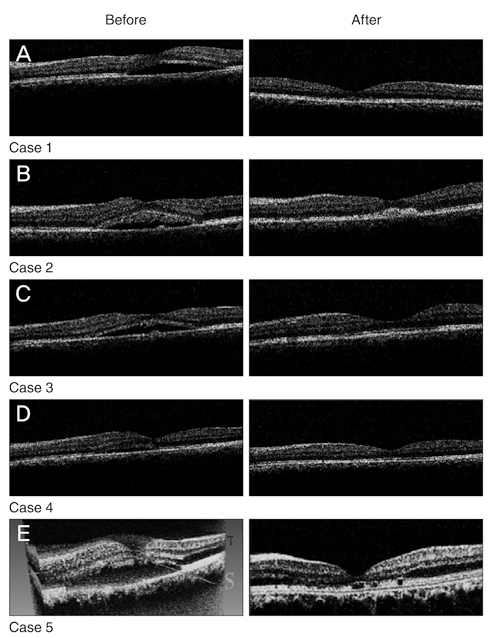
- In a clinical case series, 5 patients with not-resolved central serous choroidoretinopathy (CSC) lasting more than 1 year received one intravitreal bevacizumab injection (IVB, 1.25 mg) injection. All patients underwent a through ophthalmic examination 1 day, 1 week, and 1, 2, and 6 months after the injection. Best corrected visual acuity (BCVA) and central macular thickness were compared before and after treatment by optical coherence tomography. Mean BCVA was improved significantly (p = 0.020) from 0.60 +/- 0.25 to 0.50 +/- 0.18 and 0.29 +/- 0.19 logarithm of minimum angle of resolution at 6 and 18 weeks, respectively. Central macular thickness was also decreased significantly (p = 0.010) from 370 +/- 65 to 208 +/- 23 microm at 4 months. No recurrence was occurred during follow-up. IVB injection may have beneficial effect in the treatment of refractory CSC.
MeSH Terms
-
Adult
Angiogenesis Inhibitors/*administration & dosage
Antibodies, Monoclonal, Humanized/*administration & dosage
Central Serous Chorioretinopathy/*drug therapy/*pathology
Female
Follow-Up Studies
Humans
Intravitreal Injections
Male
Middle Aged
Tomography, Optical Coherence
Treatment Outcome
Visual Acuity/drug effects
Figure
Reference
-
1. Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmol. 2008. 86:126–145.2. Marmor MF, Tan F. Central serous chorioretinopathy: bilateral multifocal electroretinographic abnormalities. Arch Ophthalmol. 1999. 117:184–188.3. Burumcek E, Mudun A, Karacorlu S, Arslan MO. Laser photocoagulation for persistent central serous retinopathy: results of long-term follow-up. Ophthalmology. 1997. 104:616–622.4. Spitznas M. Pathogenesis of central serous retinopathy: a new working hypothesis. Graefes Arch Clin Exp Ophthalmol. 1986. 224:321–324.5. Marmor MF. New hypotheses on the pathogenesis and treatment of serous retinal detachment. Graefes Arch Clin Exp Ophthalmol. 1988. 226:548–552.6. Cardillo Piccolino F, Eandi CM, Ventre L, et al. Photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2003. 23:752–763.7. Torres-Soriano ME, Garcia-Aguirre G, Kon-Jara V, et al. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy (case reports). Graefes Arch Clin Exp Ophthalmol. 2008. 246:1235–1239.8. Von Graefe A. Ueber centrale recidivierende retinitis. Graefes Arch Clin Exp Ophthalmol. 1866. 12:211–215.9. Schaal KB, Hoeh AE, Scheuerle A, et al. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol. 2009. 19:613–617.10. Chan WM, Lam DS, Lai TY, et al. Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol. 2003. 87:1453–1458.11. Battaglia Parodi M, Da Pozzo S, Ravalico G. Photodynamic therapy in chronic central serous chorioretinopathy. Retina. 2003. 23:235–237.12. Ober MD, Yannuzzi LA, Do DV, et al. Photodynamic therapy for focal retinal pigment epithelial leaks secondary to central serous chorioretinopathy. Ophthalmology. 2005. 112:2088–2094.13. Colucciello M. Choroidal neovascularization complicating photodynamic therapy for central serous retinopathy. Retina. 2006. 26:239–242.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Intravitreal Bevacizumab and Aflibercept Injections for Central Serous Chorioretinopathy
- The Short-term Effect of Intravitreal Bevacizumab for Treatment of Central Serous Chorioretinopathy
- Factors Influencing the Effect of the Intravitreal Bevacizumab Injection in Patients with Central Serous Chorioretinopathy
- A Case of Central Serous Chorioretinopathy Associated With Retinal Detachment Improved by Intravitreal Bevacizumab Injection
- Comparison between Focal Laser Photocoagulation and Intravitreal Bevacizumab Injection for Treating Chronic Central Serous Chorioretinopathy