Ann Lab Med.
2012 Mar;32(2):133-138. 10.3343/alm.2012.32.2.133.
Comparison of Sputum and Nasopharyngeal Swab Specimens for Molecular Diagnosis of Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila
- Affiliations
-
- 1Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea. mnkim@amc.seoul.kr
- 2Division of Infectious Disease, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea.
- KMID: 1245253
- DOI: http://doi.org/10.3343/alm.2012.32.2.133
Abstract
- BACKGROUND
Differentiation of atypical pathogens is important for community-acquired pneumonia (CAP). In this study, we compared sputum and nasopharyngeal swabs (NPS) for use in detection of Mycoplasma pneumoniae (MP), Chlamydophila pneumoniae (CP), and Legionella pneumophila (LP), using Seeplex PneumoBacter ACE Detection Assay (PneumoBacter; Seegene).
METHODS
Sputum and NPS specimens were collected from patients in 15 hospitals. DNA was extracted from sputum using QIAamp DNA Stool Mini Kit (Qiagen) and from NPS using easyMAG (bioMerieux). Both types of specimens were evaluated by multiplex PCR using PneumoBacter. To determine the diagnostic performance of this assay, sputum samples were also tested using BD ProbeTec ET Atypical Pneumonia Assay (APA; Becton Dickinson).
RESULTS
Among 217 sputum and NPS, 20 (9.2%), 2 (0.9%), and 0 sputum were positive for MP, LP, and CP, respectively, whereas 8 (3.7%) NPS were positive for MP. The sputum APA test yielded 186, 206, and 204 interpretable results for MP, LP, and CP, respectively. Of these, 21 (11.3%) were positive for MP, 2 (1.0%) were positive for LP, and 0 samples were positive for CP. Compared to APA, the sensitivity and specificity of the sputum assay for MP were 95.2% and 100.0%, respectively, whereas for the NPS assay, these were 38.1% and 93.9%. Sputum testing was more sensitive than NPS testing (P=0.002). For LP and CP diagnosis, PneumoBacter and APA tests agreed 100%.
CONCLUSIONS
Specimen type is crucial and sputum is preferred over NPS for simultaneous detection of MP, LP, and CP using multiplex PCR in CAP.
Keyword
MeSH Terms
-
Chlamydophila Infections/diagnosis
Chlamydophila pneumoniae/*genetics/isolation & purification
Community-Acquired Infections/*diagnosis
DNA, Bacterial/analysis/isolation & purification
Humans
Legionella pneumophila/*genetics/isolation & purification
Legionnaires' Disease/diagnosis
Multiplex Polymerase Chain Reaction
Mycoplasma pneumoniae/*genetics/isolation & purification
Nasopharynx/*microbiology
Pneumonia, Mycoplasma/diagnosis
Reagent Kits, Diagnostic
Sputum/*microbiology
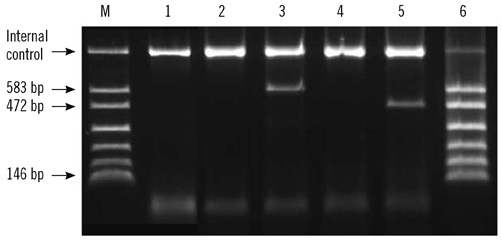
Figure
Cited by 1 articles
-
Evaluation of EuDx TM-PN MLC Detection Kit for Detection of
Mycoplasma pneumoniae, Chlamydophila pneumoniae , andLegionella pneumophila in Respiratory Specimens
Mi-Kyung Lee, Heungsup Sung, Ah Ra Cho, Hyun Young Chi
Ann Clin Microbiol. 2017;20(4):97-102. doi: 10.5145/ACM.2017.20.4.97.
Reference
-
1. Blasi F. Atypical pathogens and respiratory tract infections. Eur Respir J. 2004. 24:171–181.
Article2. Cunha BA. The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect. 2006. 12(Suppl 3):12–24.
Article3. Chong YP, Jung KS, Lee KH, Kim MN, Moon SM, Park S, et al. The bacterial etiology of community-acquired pneumonia in Korea: a nationwide prospective multicenter study. Infect Chemother. 2010. 42:397–403.
Article4. Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009. 64(Suppl 3):iii1–iii55.
Article5. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007. 44(Suppl 2):S27–S72.
Article6. Thurman KA, Warner AK, Cowart KC, Benitez AJ, Winchell JM. Detection of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella spp. in clinical specimens using a single-tube multiplex real-time PCR assay. Diagn Microbiol Infect Dis. 2011. 70:1–9.7. Socan M, Marinic-Fiser N, Kraigher A, Kotnik A, Logar M. Microbial aetiology of community-acquired pneumonia in hospitalised patients. Eur J Clin Microbiol Infect Dis. 1999. 18:777–782.8. Templeton KE, Scheltinga SA, van den Eeden WC, Graffelman AW, van den Broek PJ, Claas EC. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005. 41:345–351.
Article9. Räty R, Rönkkö E, Kleemola M. Sample type is crucial to the diagnosis of Mycoplasma pneumoniae pneumonia by PCR. J Med Microbiol. 2005. 54:287–291.10. Murdoch DR. Molecular genetic methods in the diagnosis of lower respiratory tract infections. APMIS. 2004. 112:713–727.
Article11. Strålin K, Bäckman A, Holmberg H, Fredlund H, Olcén P. Design of a multiplex PCR for Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae and Chlamydophila pneumoniae to be used on sputum samples. APMIS. 2005. 113:99–111.12. Loens K, Beck T, Ursi D, Overdijk M, Sillekens P, Goossens H, et al. Development of real-time multiplex nucleic acid sequence-based amplification for detection of Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella spp. in respiratory specimens. J Clin Microbiol. 2008. 46:185–191.13. Loens K, Van Heirstraeten L, Malhotra-Kumar S, Goossens H, Ieven M. Optimal sampling sites and methods for detection of pathogens possibly causing community-acquired lower respiratory tract infections. J Clin Microbiol. 2009. 47:21–31.
Article14. Reznikov M, Blackmore TK, Finlay-Jones JJ, Gordon DL. Comparison of nasopharyngeal aspirates and throat swab specimens in a polymerase chain reaction-based test for Mycoplasma pneumoniae. Eur J Clin Microbiol Infect Dis. 1995. 14:58–61.15. Baron EJ, Thomson RB Jr. Versalovic J, Carroll KC, editors. Specimen collection, transport, and processing: Bacteriology. Manual of clinical microbiology. 2011. 10th ed. Washington DC: ASM press;255–256.
Article16. Dorigo-Zetsma JW, Verkooyen RP, van Helden HP, van der Nat H, van den Bosch JM. Molecular detection of Mycoplasma pneumoniae in adults with community-acquired pneumonia requiring hospitalization. J Clin Microbiol. 2001. 39:1184–1186.17. Kleemola SR, Karjalainen JE, Räty RK. Rapid diagnosis of Mycoplasma pneumoniae infection: clinical evaluation of a commercial probe test. J Infect Dis. 1990. 162:70–75.18. Loens K, Beck T, Ursi D, Overdijk M, Sillekens P, Goossens H, et al. Evaluation of different nucleic acid amplification techniques for the detection of M. pneumoniae, C. pneumoniae and Legionella spp. in respiratory specimens from patients with community-acquired pneumonia. J Microbiol Methods. 2008. 73:257–262.19. Honda J, Yano T, Kusaba M, Yonemitsu J, Kitajima H, Masuoka M, et al. Clinical use of capillary PCR to diagnose Mycoplasma pneumoniae. J Clin Microbiol. 2000. 38:1382–1384.20. Brunner H. Ron E, Rottem S, editors. Adherence and pathogenicity of mycoplasma pneumoniae: a review. Microbial surface components and toxins in relation to pathogenesis. 1991. New york, NY: Plenum;81–89.
Article21. Dorigo-Zetsma JW, Zaat SA, Vriesema AJ, Dankert J. Demonstration by a nested PCR for Mycoplasma pneumoniae that M. pneumoniae load in the throat is higher in patients hospitalised for M. pneumoniae infection than in non-hospitalised subjects. J Med Microbiol. 1999. 48:1115–1122.22. Kenny GE, Kaiser GG, Cooney MK, Foy HM. Diagnosis of Mycoplasma pneumoniae pneumonia: sensitivities and specificities of serology with lipid antigen and isolation of the organism on soy peptone medium for identification of infections. J Clin Microbiol. 1990. 28:2087–2093.23. Bencini MA, van den Brule AJ, Claas EC, Hermans MH, Melchers WJ, Noordhoek GT, et al. Multicenter comparison of molecular methods for detection of Legionella spp. in sputum samples. J Clin Microbiol. 2007. 45:3390–3392.24. Hernes SS, Quarsten H, Hagen E, Lyngroth AL, Pripp AH, Bjorvatn B, et al. Swabbing for respiratory viral infections in older patients: a comparison of rayon and nylon flocked swabs. Eur J Clin Microbiol Infect Dis. 2011. 30:159–165.
Article25. Edelstein PH. Versalovic J, Carroll KC, et al. Legionella. Manual of Clinical Microbiology. 2011. 10th ed. Washington DC: ASM press;774–775.
Article26. Den Boer JW, Yzerman EP. Diagnosis of Legionella infection in Legionnaires' disease. Eur J Clin Microbiol Infect Dis. 2004. 23:871–878.
Article27. McDonough EA, Metzgar D, Hansen CJ, Myers CA, Russell KL. A cluster of Legionella-associated pneumonia cases in a population of military recruits. J Clin Microbiol. 2007. 45:2075–2077.28. Ramirez JA, Ahkee S, Tolentino A, Miller RD, Summersgill JT. Diagnosis of Legionella pneumophila, Mycoplasma pneumoniae, or Chlamydia pneumoniae lower respiratory infection using the polymerase chain reaction on a single throat swab specimen. Diagn Microbiol Infect Dis. 1996. 24:7–14.29. Diederen BM, Peeters MF. Are oropharyngeal swabs suitable as samples for Legionella-specific PCR testing? J Clin Microbiol. 2007. 45:3482–3483.30. Gaydos C, Essig A. Versalovic J, Carroll KC, editors. Chlamydiaceae. Manual of Clinical Microbiology. 2011. 10th ed. Washington DC: ASM press;990–991.
Article31. Boman J, Allard A, Persson K, Lundborg M, Juto P, Wadell G. Rapid diagnosis of respiratory Chlamydia pneumoniae infection by nested touchdown polymerase chain reaction compared with culture and antigen detection by EIA. J Infect Dis. 1997. 175:1523–1526.32. Kuoppa Y, Boman J, Scott L, Kumlin U, Eriksson I, Allard A. Quantitative detection of respiratory Chlamydia pneumoniae infection by realtime PCR. J Clin Microbiol. 2002. 40:2273–2274.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Sputum and Nasopharyngeal Aspirates for Molecular Detection of Community-Acquired Pneumonia Pathogens
- Mycoplasma pneumoniae Pneumonia Unresponsive to Macrolide Treatment
- Evaluation of EuDxâ„¢-PN MLC Detection Kit for Detection of Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila in Respiratory Specimens
- Current perspectives on atypical pneumonia in children
- Clinical Evaluation of the Multiplex PCR Assay for the Detection of Bacterial Pathogens in Respiratory Specimens from Patients with Pneumonia