J Korean Med Sci.
2011 Nov;26(11):1495-1500. 10.3346/jkms.2011.26.11.1495.
Protective Effect of Hypoxic Preconditioning on Hypoxic-Ischemic Injured Newborn Rats
- Affiliations
-
- 1Department of Pediatrics, Hanyang University College of Medicine, Seoul, Korea.
- 2Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. kskim@amc.seoul.kr
- 3NMR Laboratory, Asan Institute for Life Science, Seoul, Korea.
- KMID: 1123451
- DOI: http://doi.org/10.3346/jkms.2011.26.11.1495
Abstract
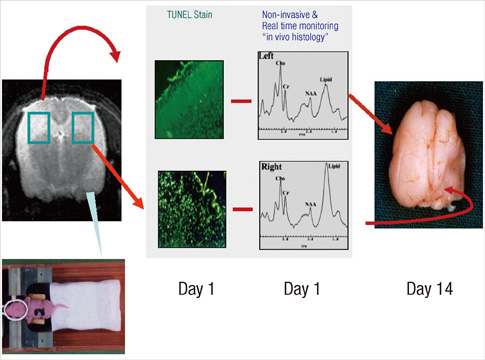
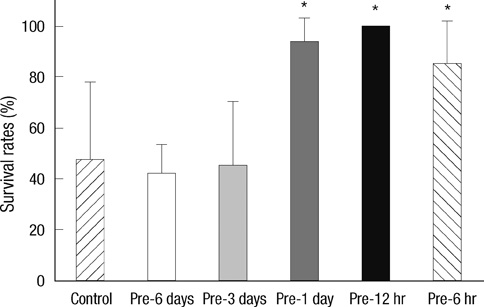
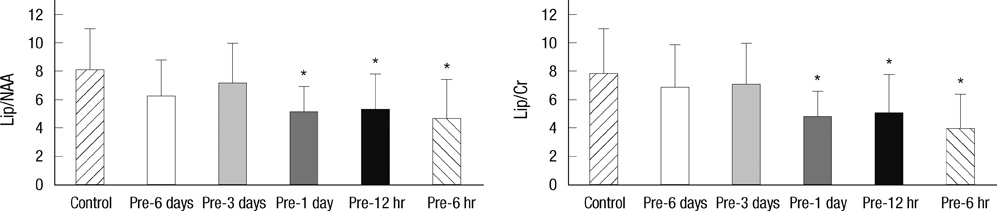
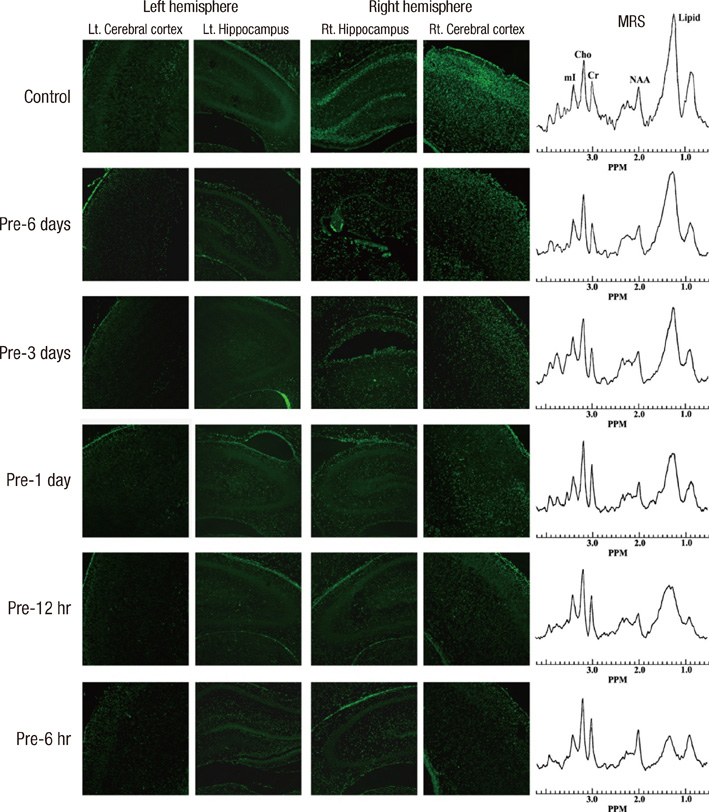
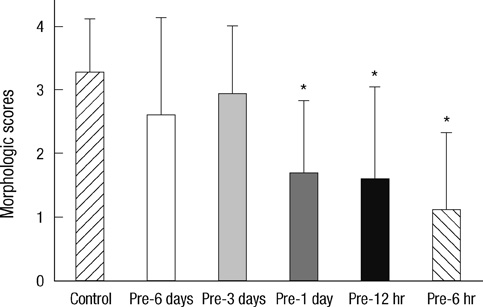
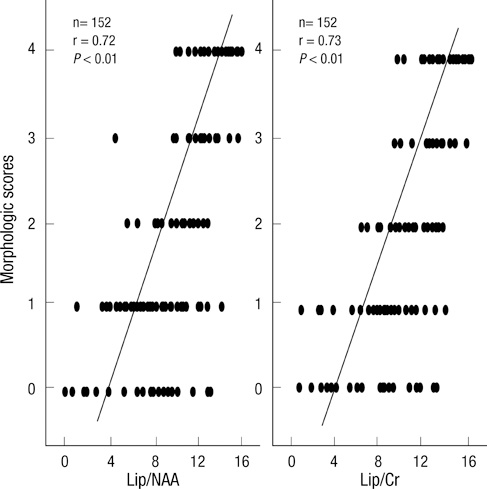
- Brief episodes of cerebral hypoxia-ischemia cause transient ischemic tolerance to subsequent ischemic events that are otherwise lethal. This study was conducted to evaluate the protective effect of hypoxic preconditioning on hypoxic-ischemic injury in the neonatal rat and the persistence of a protective window after hypoxic preconditioning. The rats were preconditioned with hypoxia (8% oxygen, 92% nitrogen) for three hours, subjected to ischemia using ligation of the right common carotid artery, and then exposed to another three hours of hypoxia. Using proton magnetic resonance spectroscopy, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling (TUNEL) staining, and morphologic scores, this study shows that hypoxic preconditioning 6-hr to 1-day before hypoxic-ischemic injury increases survival rates and has neuroprotective effects against subsequent hypoxic-ischemic injury. The mechanism of the protective effects of hypoxic preconditioning in the newborn rat brain may involve downregulation of apoptotic cell death.
MeSH Terms
-
Animals
Animals, Newborn
Apoptosis
Aspartic Acid/analogs & derivatives/analysis
Brain/metabolism/pathology
Carotid Arteries/surgery
Creatine/analysis
Hypoxia-Ischemia, Brain/metabolism/pathology/*physiopathology
In Situ Nick-End Labeling
Ischemic Preconditioning/*methods
Magnetic Resonance Spectroscopy
Rats
Rats, Sprague-Dawley
Survival Rate
Figure
Reference
-
1. Lee JM, Grabb MC, Zipfel GJ, Choi DW. Brain tissue responses to ischemia. J Clin Invest. 2000. 106:723–731.2. Pin TW, Eldridge B, Galea MP. A review of developmental outcomes of term infants with post-asphyxia neonatal encephalopathy. Eur J Paediatr Neurol. 2009. 13:224–234.3. Cantagrel S, Krier C, Ducrocq S, Bodard S, Payen V, Laugier J, Guilloteau D, Chalon S. Hypoxic preconditioning reduces apoptosis in a rat model of immature brain hypoxia-ischaemia. Neurosci Lett. 2003. 347:106–110.4. Sanders RD, Manning HJ, Robertson NJ, Ma D, Edwards AD, Hagberg H, Maze M. Preconditioning and postinsult therapies for perinatal hypoxic-ischemic injury at term. Anesthesiology. 2010. 113:233–249.5. Gidday JM, Fitzgibbons JC, Shah AR, Park TS. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci Lett. 1994. 168:221–224.6. Cadenas S, Aragonés J, Landázuri MO. Mitochondrial reprogramming through cardiac oxygen sensors in ischaemic heart disease. Cardiovasc Res. 2010. 88:219–228.7. Wrang ML, Moller F, Alsbo CW, Diemer NH. Changes in gene expression following induction of ischemic tolerance in rat brain: detection and verification. J Neurosci Res. 2001. 65:54–58.8. Miller BA, Perez RS, Shah AR, Gonzales ER, Park TS, Gidday JM. Cerebral protection by hypoxic preconditioning in a murine model of focal ischemia-reperfusion. Neuroreport. 2001. 12:1663–1669.9. Palmer C, Vannucci RC, Towfighi J. Reduction of perinatal hypoxic-ischemic brain damage with allopurinol. Pediatr Res. 1990. 27:332–336.10. Sivaswamy S, Neafsey EJ, Collins MA. Neuroprotective preconditioning of rat brain cultures with ethanol: potential transduction by PKC isoforms and focal adhesion kinase upstream of increases in effector heat shock proteins. Eur J Neurosci. 2010. 32:1800–1812.11. Nishio S, Chen ZF, Yunoki M, Toyoda T, Anzivino M, Lee KS. Hypothermia-induced ischemic tolerance. Ann N Y Acad Sci. 1999. 890:26–41.12. Schurr A, Payne RS, Tseng MT, Gozal E, Gozal D. Excitotoxic preconditioning elicited by both glutamate and hypoxia and abolished by lactate transport inhibition in rat hippocampal slices. Neurosci Lett. 2001. 307:151–154.13. Khaspekov L, Shamloo M, Victorov I, Wieloch T. Sublethal in vitro glucose-oxygen deprivation protects cultured hippocampal neurons against a subsequent severe insult. Neuroreport. 1998. 9:1273–1276.14. Meloni BP, Majda BT, Knuckey NW. Evaluation of preconditioning treatments to protect near-pure cortical neuronal cultures from in vitro ischemia induced acute and delayed neuronal death. Brain Res. 2002. 928:69–75.15. Liu J, Ginis I, Spatz M, Hallenbeck JM. Hypoxic preconditioning protects cultured neurons against hypoxic stress via TNF-alpha and ceramide. Am J Physiol Cell Physiol. 2000. 278:C144–C153.16. Rybnikova E, Vataeva L, Tyulkova E, Gluschenko T, Otellin V, Pelto-Huikko M, Samoilov MO. Mild hypoxia preconditioning prevents impairment of passive avoidance learning and suppression of brain NGFI-A expression induced by severe hypoxia. Behav Brain Res. 2005. 160:107–114.17. Hieber S, Huhn R, Hollmann MW, Weber NC, Preckel B. Hypoxia-inducible factor 1 and related gene products in anaesthetic-induced preconditioning. Eur J Anaesthesiol. 2009. 26:201–206.18. Blondeau N, Widmann C, Lazdunski M, Heurteaux C. Activation of the nuclear factor-kappaB is a key event in brain tolerance. J Neurosci. 2001. 21:4668–4677.19. Lee HT, Chang YC, Wang LY, Wang ST, Huang CC, Ho CJ. cAMP response element-binding protein activation in ligation preconditioning in neonatal brain. Ann Neurol. 2004. 56:611–623.20. Wang X, Deng J, Boyle DW, Zhong J, Lee WH. Potential role of IGF-I in hypoxia tolerance using a rat hypoxic-ischemic model: activation of hypoxia-inducible factor 1alpha. Pediatr Res. 2004. 55:385–394.21. Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K, Kamada T. 'Ischemic tolerance' phenomenon found in the brain. Brain Res. 1990. 528:21–24.22. Yin XH, Zhang QG, Miao B, Zhang GY. Neuroprotective effects of preconditioning ischaemia on ischaemic brain injury through inhibition of mixed-lineage kinase 3 via NMDA receptor-mediated Akt1 activation. J Neurochem. 2005. 93:1021–1029.23. Pugliese AM, Latini S, Corradetti R, Pedata F. Brief, repeated, oxygen-glucose deprivation episodes protect neurotransmission from a longer ischemic episode in the in vitro hippocampus: role of adenosine receptors. Br J Pharmacol. 2003. 140:305–314.24. Arthur PG, Lim SC, Meloni BP, Munns SE, Chan A, Knuckey NW. The protective effect of hypoxic preconditioning on cortical neuronal cultures is associated with increases in the activity of several antioxidant enzymes. Brain Res. 2004. 1017:146–154.25. Nakajima W, Ishida A, Lange MS, Gabrielson KL, Wilson MA, Martin LJ, Blue ME, Johnston MV. Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat. J Neurosci. 2000. 20:7994–8004.26. Li YK, Liu GR, Zhou XG, Cai AQ. Experimental hypoxic-ischemic encephalopathy: comparison of apparent diffusion coefficients and proton magnetic resonance spectroscopy. Magn Reson Imaging. 2010. 28:487–494.27. Hüppi PS, Lazeyras F. Proton magnetic resonance spectroscopy ((1)HMRS) in neonatal brain injury. Pediatr Res. 2001. 49:317–320.28. da Silva LF, Höefel Filho JR, Anés M, Nunes ML. Prognostic value of 1HMRS in neonatal encephalopathy. Pediatr Neurol. 2006. 34:360–366.29. Blankenberg FG, Katsikis PD, Storrs RW, Beaulieu C, Spielman D, Chen JY, Naumovski L, Tait JF. Quantitative analysis of apoptotic cell death using proton nuclear magnetic resonance spectroscopy. Blood. 1997. 89:3778–3786.30. Park SJ, Yoon HS, Lim KH, Lee JH, Kim KS, Pi SY. Early prediction of hypoxic-ischemic brain damage in newbon rats using proton magnetic resonance spectroscopy and neuroprotective effect of EGb 761. J Korean Soc Neonatol. 2001. 8:133–140.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Hypoxic-Preconditioning on the Reperfusion-Induced Arrhythmias in the Cat Hearts
- The Protective Effect of Ischemic and Hypoxic Preconditioning on Hypoxic-ischemic Brain Injury in the Neonatal Rat: 1H Magnetic Resonance Spectroscopic Study
- The effect of mitochondrial adenosine triphosphate-sensitive potassium (K(ATP)) channel blocker on ischemic preconditioning in hypoxic-ischemic brain injury model of neonatal rat
- Ischemic Preconditioning and the Role of Protein Kinase C in Cultured Retinal Ganglion Cell Line
- Neuroprotective Effect of Growth Hormone in Neonatal Rat with Hypoxic Ischemic Brain Injury