J Korean Med Sci.
2011 Nov;26(11):1428-1438. 10.3346/jkms.2011.26.11.1428.
Genes Associated with Recurrence of Hepatocellular Carcinoma: Integrated Analysis by Gene Expression and Methylation Profiling
- Affiliations
-
- 1Miles and Shirley Fiterman Center for Digestive Diseases, College of Medicine, Mayo Clinic, Rochester, MN, USA. kjkang@dsmc.or.kr
- 2Department of Biological Sciences, Dong-A University, Busan, Korea.
- 3Department of Surgery, Keimyung University School of Medicine, Daegu, Korea.
- 4Division of Biomedical Statistics and Informatics, College of Medicine, Mayo Clinic, Rochester, MN, USA.
- 5Department of Systems Biology, The University of Texas M. D. Anderson Cancer Center, Houston, TX, USA.
- 6Laboratory of Experimental Carcinogenesis, National Cancer Institute, Bethesda, MD, USA.
- 7Korean Bioinformation Center, Korea Research Institute of Bioscience & Biotechnology, Daejeon, Korea.
- KMID: 1123442
- DOI: http://doi.org/10.3346/jkms.2011.26.11.1428
Abstract
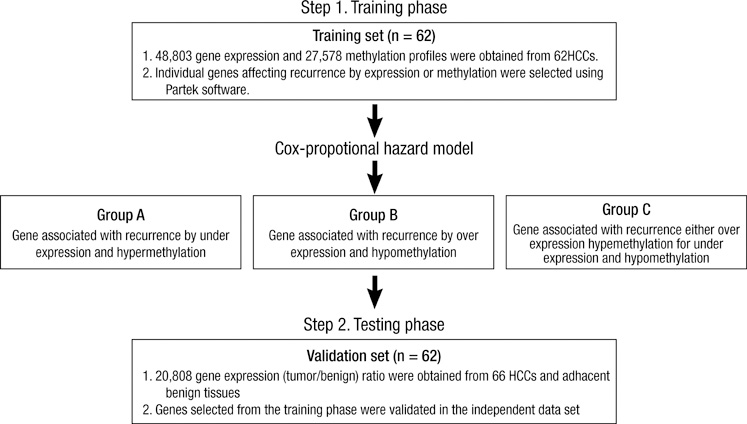
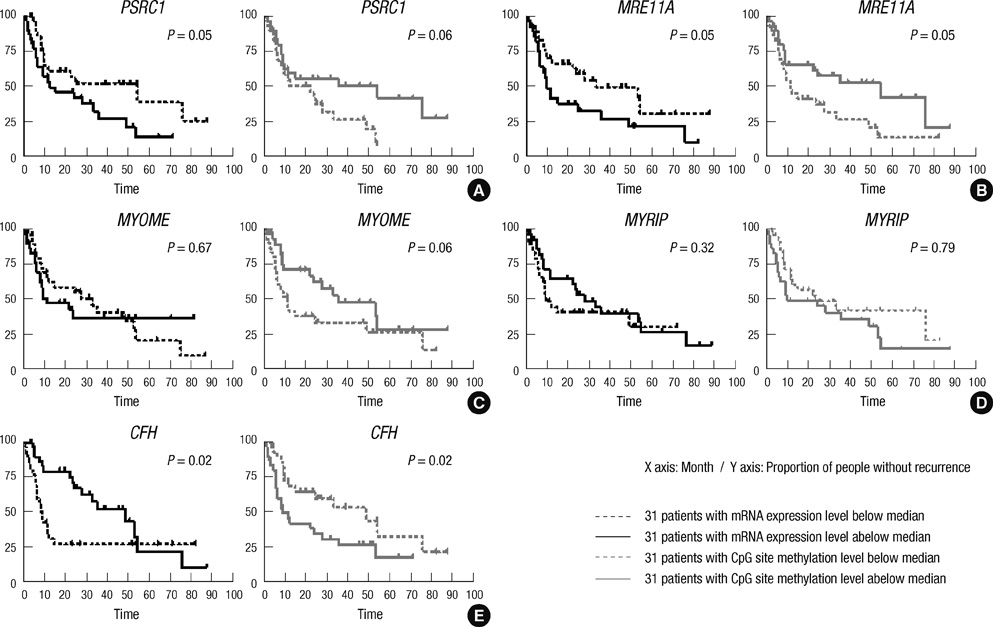
- Gene expression is suppressed by DNA methylation. The goal of this study was to identify genes whose CpG site methylation and mRNA expression are associated with recurrence after surgical resection for hepatocellular carcinoma (HCC). Sixty-two HCCs were examined by both whole genome DNA methylation and transcriptome analysis. The Cox model was used to select genes associated with recurrence. A validation was performed in an independent cohort of 66 HCC patients. Among fifty-nine common genes, increased CpG site methylation and decreased mRNA expression were associated with recurrence for 12 genes (Group A), whereas decreased CpG site methylation and increased mRNA expression were associated with recurrence for 25 genes (Group B). The remaining 22 genes were defined as Group C. Complement factor H (CFH) and myosin VIIA and Rab interacting protein (MYRIP) in Group A; proline/serine-rich coiled-coil 1 (PSRC1), meiotic recombination 11 homolog A (MRE11A), and myosin IE (MYO1E) in Group B; and autophagy-related protein LC3 A (MAP1LC3A), and NADH dehydrogenase 1 alpha subcomplex assembly factor 1 (NDUFAF1) in Group C were validated. In conclusion, potential tumor suppressor (CFH, MYRIP) and oncogenes (PSRC1, MRE11A, MYO1E) in HCC are reported. The regulation of individual genes by methylation in hepatocarcinogenesis needs to be validated.
Keyword
MeSH Terms
-
Adult
Aged
Carcinoma, Hepatocellular/*genetics/pathology/surgery
CpG Islands
*DNA Methylation
Female
Gene Expression Profiling
Gene Expression Regulation, Neoplastic
Humans
Liver/pathology
Liver Neoplasms/*genetics/pathology/surgery
Male
Middle Aged
Neoplasm Recurrence, Local/*genetics
Oligonucleotide Array Sequence Analysis
Proportional Hazards Models
RNA, Messenger/biosynthesis
Transcriptome/genetics
Figure
Cited by 1 articles
-
Experiment of Ischemia/Reperfusion Injury of the Cirrhotic Liver in the Murine Mouse Model
Koo Jeong Kang
Hanyang Med Rev. 2013;33(3):142-149. doi: 10.7599/hmr.2013.33.3.142.
Reference
-
1. Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010. 7:448–458.2. Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002. 3:415–428.3. Coulouarn C, Factor VM, Thorgeirsson SS. Transforming growth factor-beta gene expression signature in mouse hepatocytes predicts clinical outcome in human cancer. Hepatology. 2008. 47:2059–2067.4. Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z, Nevens F, Roskams T, Thorgeirsson SS. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006. 12:410–416.5. Arai E, Ushijima S, Gotoh M, Ojima H, Kosuge T, Hosoda F, Shibata T, Kondo T, Yokoi S, Imoto I, Inazawa J, Hirohashi S, Kanai Y. Genome-wide DNA methylation profiles in liver tissue at the precancerous stage and in hepatocellular carcinoma. Int J Cancer. 2009. 125:2854–2862.6. Shin SH, Kim BH, Jang JJ, Suh KS, Kang GH. Identification of novel methylation markers in hepatocellular carcinoma using a methylation array. J Korean Med Sci. 2010. 25:1152–1159.7. Yang JD, Sun Z, Hu C, Lai J, Dove R, Nakamura I, Lee JS, Thorgeirsson SS, Kang KJ, Chu IS, Roberts LR. Sulfatase 1 and sulfatase 2 in hepatocellular carcinoma: associated signaling pathways, tumor phenotypes, and survival. Genes Chromosomes Cancer. 2011. 50:122–135.8. Fan JB, Gunderson KL, Bibikova M, Yeakley JM, Chen J, Wickham Garcia E, Lebruska LL, Laurent M, Shen R, Barker D. Illumina universal bead arrays. Methods Enzymol. 2006. 410:57–73.9. Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol. 2011. 21:35–43.10. Hsieh WJ, Hsieh SC, Chen CC, Wang FF. Human DDA3 is an oncoprotein down-regulated by p53 and DNA damage. Biochem Biophys Res Commun. 2008. 369:567–572.11. Hsieh PC, Chang JC, Sun WT, Hsieh SC, Wang MC, Wang FF. p53 downstream target DDA3 is a novel microtubule-associated protein that interacts with end-binding protein EB3 and activates beta-catenin pathway. Oncogene. 2007. 26:4928–4940.12. Sun WT, Hsieh PC, Chiang ML, Wang MC, Wang FF. p53 target DDA3 binds ASPP2 and inhibits its stimulation on p53-mediated BAX activation. Biochem Biophys Res Commun. 2008. 376:395–398.13. Oh BK, Kim H, Park HJ, Shim YH, Choi J, Park C, Park YN. DNA methyltransferase expression and DNA methylation in human hepatocellular carcinoma and their clinicopathological correlation. Int J Mol Med. 2007. 20:65–73.14. Yuan Y, Wang J, Li J, Wang L, Li M, Yang Z, Zhang C, Dai JL. Frequent epigenetic inactivation of spleen tyrosine kinase gene in human hepatocellular carcinoma. Clin Cancer Res. 2006. 12:6687–6695.15. Shivapurkar N, Stastny V, Okumura N, Girard L, Xie Y, Prinsen C, Thunnissen FB, Wistuba II, Czerniak B, Frenkel E, Roth JA, Liloglou T, Xinarianos G, Field JK, Minna JD, Gazdar AF. Cytoglobin, the newest member of the globin family, functions as a tumor suppressor gene. Cancer Res. 2008. 68:7448–7456.16. Man KN, Philipsen S, Tan-Un KC. Localization and expression pattern of cytoglobin in carbon tetrachloride-induced liver fibrosis. Toxicol Lett. 2008. 183:36–44.17. Xu R, Harrison PM, Chen M, Li L, Tsui TY, Fung PC, Cheung PT, Wang G, Li H, Diao Y, Krissansen GW, Xu S, Farzaneh F. Cytoglobin overexpression protects against damage-induced fibrosis. Mol Ther. 2006. 13:1093–1100.18. Sidiropoulos M, Pampalakis G, Sotiropoulou G, Katsaros D, Diamandis EP. Downregulation of human kallikrein 10 (KLK10/NES1) by CpG island hypermethylation in breast, ovarian and prostate cancers. Tumour Biol. 2005. 26:324–336.19. Kioulafa M, Kaklamanis L, Stathopoulos E, Mavroudis D, Georgoulias V, Lianidou ES. Kallikrein 10 (KLK10) methylation as a novel prognostic biomarker in early breast cancer. Ann Oncol. 2009. 20:1020–1025.20. Lu CY, Hsieh SY, Lu YJ, Wu CS, Chen LC, Lo SJ, Wu CT, Chou MY, Huang TH, Chang YS. Aberrant DNA methylation profile and frequent methylation of KLK10 and OXGR1 genes in hepatocellular carcinoma. Genes Chromosomes Cancer. 2009. 48:1057–1068.21. Moreno-Navarrete JM, Martínez-Barricarte R, Catalán V, Sabater M, Gómez-Ambrosi J, Ortega FJ, Ricart W, Blüher M, Frühbeck G, Rodríguez de Cordoba S, Fernández-Real JM. Complement factor H is expressed in adipose tissue in association with insulin resistance. Diabetes. 2010. 59:200–209.22. Linton KM, Hey Y, Saunders E, Jeziorska M, Denton J, Wilson CL, Swindell R, Dibben S, Miller CJ, Pepper SD, Radford JA, Freemont AJ. Acquisition of biologically relevant gene expression data by Affymetrix microarray analysis of archival formalin-fixed paraffin-embedded tumours. Br J Cancer. 2008. 98:1403–1414.23. Stewart GS, Maser RS, Stankovic T, Bressan DA, Kaplan MI, Jaspers NG, Raams A, Byrd PJ, Petrini JH, Taylor AM. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell. 1999. 99:577–587.24. Bartkova J, Tommiska J, Oplustilova L, Aaltonen K, Tamminen A, Heikkinen T, Mistrik M, Aittomäki K, Blomqvist C, Heikkilä P, Lukas J, Nevanlinna H, Bartek J. Aberrations of the MRE11-RAD50-NBS1 DNA damage sensor complex in human breast cancer: MRE11 as a candidate familial cancer-predisposing gene. Mol Oncol. 2008. 2:296–316.25. Sivridis E, Koukourakis MI, Zois CE, Ledaki I, Ferguson DJ, Harris AL, Gatter KC, Giatromanolaki A. LC3A-positive light microscopy detected patterns of autophagy and prognosis in operable breast carcinomas. Am J Pathol. 2010. 176:2477–2489.26. Sivridis E, Giatromanolaki A, Liberis V, Koukourakis MI. Autophagy in endometrial carcinomas and prognostic relevance of 'stone-like' structures (SLS): what is destined for the atypical endometrial hyperplasia? Autophagy. 2011. 7:74–82.27. Krendel M, Osterweil EK, Mooseker MS. Myosin 1E interacts with synaptojanin-1 and dynamin and is involved in endocytosis. FEBS Lett. 2007. 581:644–650.28. Klomp AE, Teofilo K, Legacki E, Williams DS. Analysis of the linkage of MYRIP and MYO7A to melanosomes by RAB27A in retinal pigment epithelial cells. Cell Motil Cytoskeleton. 2007. 64:474–487.29. Nightingale TD, Pattni K, Hume AN, Seabra MC, Cutler DF. Rab27a and MyRIP regulate the amount and multimeric state of VWF released from endothelial cells. Blood. 2009. 113:5010–5018.30. Vogel RO, Janssen RJ, Ugalde C, Grovenstein M, Huijbens RJ, Visch HJ, van den Heuvel LP, Willems PH, Zeviani M, Smeitink JA, Nijtmans LG. Human mitochondrial complex I assembly is mediated by NDUFAF1. FEBS J. 2005. 272:5317–5326.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of Novel Methylation Markers in Hepatocellular Carcinoma using a Methylation Array
- Methylation of p16 and E-cadherin in ameloblastoma
- DNA Methylation in Development
- Integrative Analysis of Microarray Data to Reveal Regulation Patterns in the Pathogenesis of Hepatocellular Carcinoma
- In silico Identification of SFRP1 as a Hypermethylated Gene in Colorectal Cancers