Korean J Ophthalmol.
2012 Feb;26(1):45-48. 10.3341/kjo.2012.26.1.45.
The Role of Focal Adhesion Kinase in the TGF-beta-Induced Myofibroblast Transdifferentiation of Human Tenon's Fibroblasts
- Affiliations
-
- 1Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea. shhan222@yuhs.ac
- KMID: 1120181
- DOI: http://doi.org/10.3341/kjo.2012.26.1.45
Abstract
- PURPOSE
To investigate the role of focal adhesion kinase (FAK) in transforming growth factor (TGF)-beta-induced myofibroblast transdifferentiation of human Tenon's fibroblasts.
METHODS
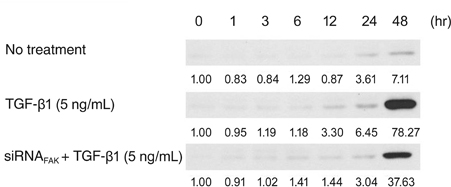
Primary cultured human Tenon's fibroblasts were exposed to TGF-beta1 for up to 48 hours. The mRNA levels of FAK, alpha smooth muscle actin (alphaSMA), and beta-actin were determined by quantitative real time reverse transcription polymerase chain reaction. The protein levels of collagen type I, FAK, phospho-FAK, alphaSMA, and beta-actin were determined by Western immunoblots. After the small interfering RNA targeting FAK (siRNA(FAK)) molecules were delivered into the cells, the expressions of alphaSMA proteins were determined by Western immunoblots.
RESULTS
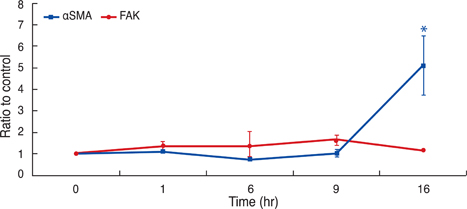
In human Tenon's fibroblasts, TGF-beta1 significantly increased the mRNA and protein expressions of alphaSMA. However, when the action of FAK was inhibited using siRNAFAK, the TGF-beta1-induced expression of alphaSMA was attenuated.
CONCLUSIONS
Our data suggest that FAK may be associated with the TGF-beta1-induced transdifferentiation of human Tenon's fibroblasts to myofibroblasts, which is the essential step of subconjunctival fibrosis.
Keyword
MeSH Terms
-
Actins/metabolism
Analysis of Variance
Blotting, Western
Cell Transdifferentiation/drug effects
Cells, Cultured
Collagen/metabolism
Fibroblasts/cytology/drug effects/metabolism
Focal Adhesion Protein-Tyrosine Kinases/*metabolism
Humans
Myofibroblasts
RNA, Messenger/metabolism
RNA, Small Interfering/metabolism
Real-Time Polymerase Chain Reaction
Transforming Growth Factor beta/*pharmacology
Figure
Reference
-
1. Eyden B. The myofibroblast: an assessment of controversial issues and a definition useful in diagnosis and research. Ultrastruct Pathol. 2001. 25:39–50.2. Eyden B. Electron microscopy in the study of myofibroblastic lesions. Semin Diagn Pathol. 2003. 20:13–24.3. Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004. 48:509–517.4. Meyer-Ter-Vehn T, Grehn F, Schlunck G. Localization of TGF-beta type II receptor and ED-A fibronectin in normal conjunctiva and failed filtering blebs. Mol Vis. 2008. 14:136–141.5. Saika S, Yamanaka O, Okada Y, et al. TGF beta in fibroproliferative diseases in the eye. Front Biosci (Schol Ed). 2009. 1:376–390.6. Seong GJ, Hong S, Jung SA, et al. TGF-beta-induced interleukin-6 participates in transdifferentiation of human Tenon’s fibroblasts to myofibroblasts. Mol Vis. 2009. 15:2123–2128.7. Nakamura H, Siddiqui SS, Shen X, et al. RNA interference targeting transforming growth factor-beta type II receptor suppresses ocular inflammation and fibrosis. Mol Vis. 2004. 10:703–711.8. Meyer-Ter-Vehn T, Gebhardt S, Sebald W, et al. p38 inhibitors prevent TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci. 2006. 47:1500–1509.9. Yamanaka O, Saika S, Ikeda K, et al. Interleukin-7 modulates extracellular matrix production and TGF-beta signaling in cultured human subconjunctival fibroblasts. Curr Eye Res. 2006. 31:491–499.10. Meyer-Ter-Vehn T, Katzenberger B, Han H, et al. Lovastatin inhibits TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci. 2008. 49:3955–3960.11. Chatzizacharias NA, Kouraklis GP, Theocharis SE. The role of focal adhesion kinase in early development. Histol Histopathol. 2010. 25:1039–1055.12. Schwock J, Dhani N, Hedley DW. Targeting focal adhesion kinase signaling in tumor growth and metastasis. Expert Opin Ther Targets. 2010. 14:77–94.13. Thannickal VJ, Lee DY, White ES, et al. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003. 278:12384–12389.14. Mimura Y, Ihn H, Jinnin M, et al. Constitutive phosphorylation of focal adhesion kinase is involved in the myofibroblast differentiation of scleroderma fibroblasts. J Invest Dermatol. 2005. 124:886–892.15. Brenmoehl J, Miller SN, Hofmann C, et al. Transforming growth factor-beta 1 induces intestinal myofibroblast differentiation and modulates their migration. World J Gastroenterol. 2009. 15:1431–1442.16. McLean GW, Carragher NO, Avizienyte E, et al. The role of focal-adhesion kinase in cancer: a new therapeutic opportunity. Nat Rev Cancer. 2005. 5:505–515.17. Cox BD, Natarajan M, Stettner MR, Gladson CL. New concepts regarding focal adhesion kinase promotion of cell migration and proliferation. J Cell Biochem. 2006. 99:35–52.18. Liu S, Xu SW, Kennedy L, et al. FAK is required for TGF-beta-induced JNK phosphorylation in fibroblasts: implications for acquisition of a matrix-remodeling phenotype. Mol Biol Cell. 2007. 18:2169–2178.19. Horowitz JC, Rogers DS, Sharma V, et al. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007. 19:761–771.20. Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007. 22:Suppl 1. S79–S84.21. Walsh MF, Ampasala DR, Hatfield J, et al. Transforming growth factor-beta stimulates intestinal epithelial focal adhesion kinase synthesis via Smad- and p38-dependent mechanisms. Am J Pathol. 2008. 173:385–399.22. Walsh MF, Ampasala DR, Rishi AK, Basson MD. TGF-beta1 modulates focal adhesion kinase expression in rat intestinal epithelial IEC-6 cells via stimulatory and inhibitory Smad binding elements. Biochim Biophys Acta. 2009. 1789:88–98.23. Cordeiro MF. Technology evaluation: lerdelimumab, Cambridge Antibody Technology. Curr Opin Mol Ther. 2003. 5:199–203.24. Mead AL, Wong TT, Cordeiro MF, et al. Evaluation of anti-TGF-beta2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest Ophthalmol Vis Sci. 2003. 44:3394–3401.25. CAT-152 0102 Trabeculectomy Study Group. Khaw P, Grehn F, et al. A phase III study of subconjunctival human anti-transforming growth factor beta(2) monoclonal antibody (CAT-152) to prevent scarring after first-time trabeculectomy. Ophthalmology. 2007. 114:1822–1830.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory Effects of Imatinib Mesylate on Transdifferentiation of Lens Epithelial Cells into Fibroblast
- Role of Hydrogen Sulfide in the Survival of Fibroblasts and Fibroblast-mediated Contraction of Collagen Gel
- Role of Reactive Oxygen Species in Transforming Growth Factor - beta1 - inuduced Fibronectin Secretion and alpha - Smooth Muscle Actin Expression in Human Lung Fibroblasts
- Effects of Prednisolone and Loteprednol Eyedrops on the Proliferation of Human Tenon's Capsule Fibroblasts
- Vactosertib, a Novel, Orally Bioavailable Activin Receptor-Like Kinase 5 Inhibitor, Promotes Regression of Fibrotic Plaques in a Rat Model of Peyronie’s Disease