J Korean Med Sci.
2012 Feb;27(2):160-169. 10.3346/jkms.2012.27.2.160.
Comparison of Early and Late Conversion of Sirolimus in Experimental Model of Chronic Cyclosporine Nephropathy
- Affiliations
-
- 1Transplant Research Center, Convergent Research Consortium for Immunologic Disease, The Catholic University of Korea, Seoul, Korea. yangch@catholic.ac.kr
- 2Cell Death Disease Research Center, Department of Anatomy, The Catholic University of Korea, Seoul, Korea.
- KMID: 1120153
- DOI: http://doi.org/10.3346/jkms.2012.27.2.160
Abstract
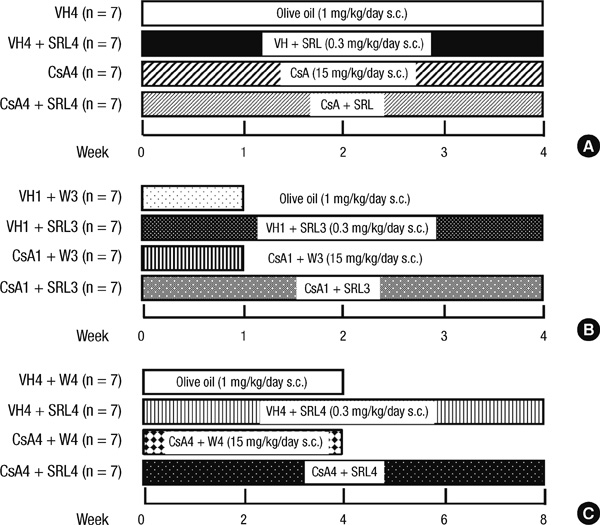
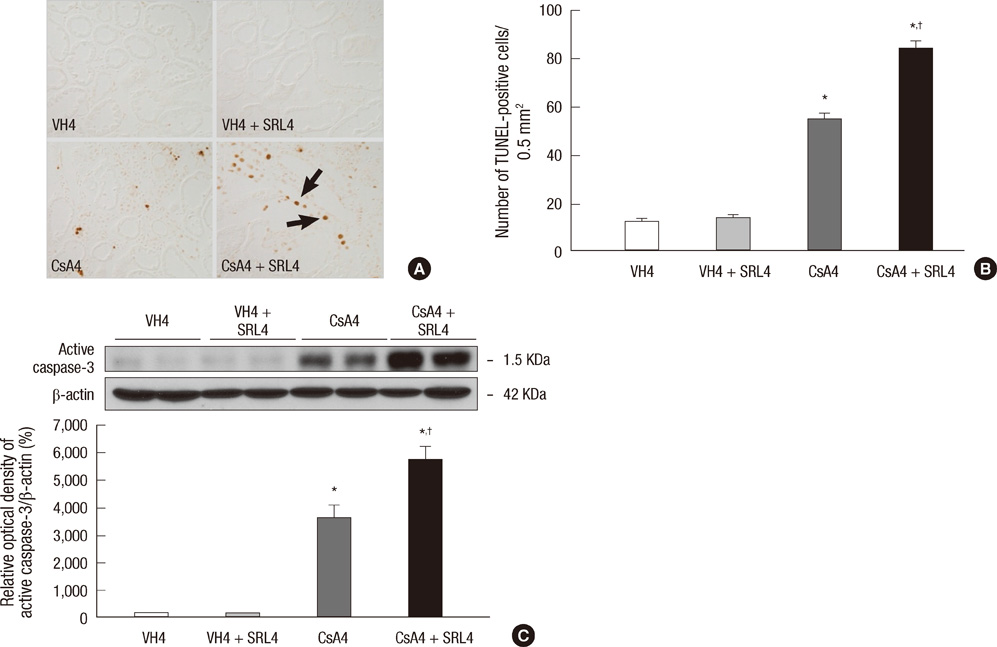
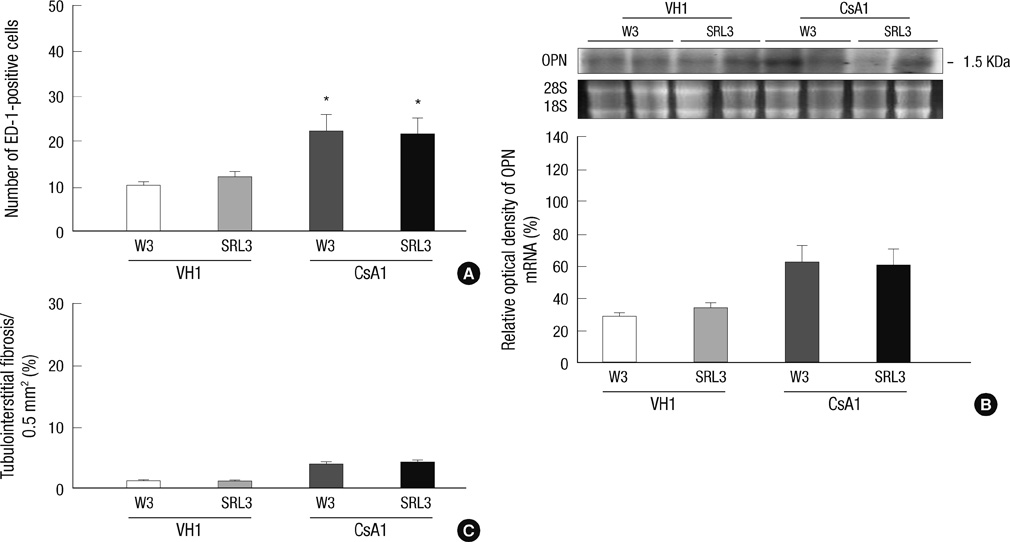
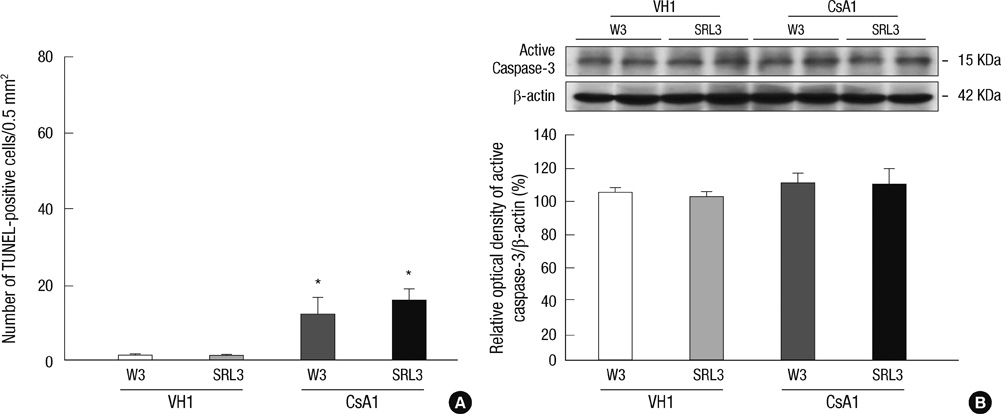
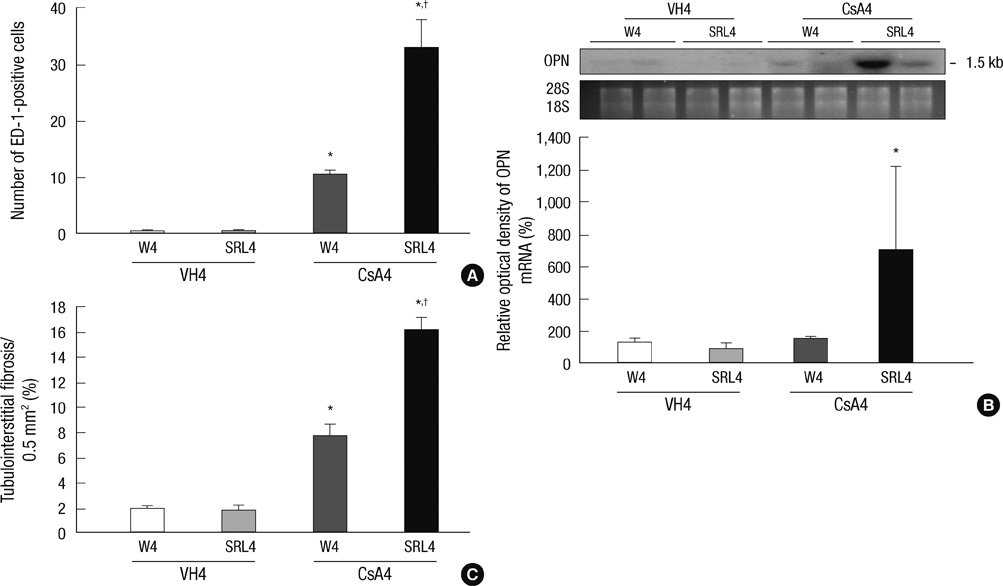
- Sirolimus (SRL) is a promising drug for replacing calcineurin inhibitors. We performed this study to determine the optimal time of conversion from cyclosporine (CsA) to SRL in an experimental model of chronic CsA nephropathy. Three separate studies were performed. In the first study, SRL was given to rats with or without CsA for 4 weeks. In the second study, rats were treated initially with CsA for 1 week, and then switched to SRL (early conversion). In the third study, CsA was given for 4 weeks and then replaced by SRL for 4 weeks treatment of CsA (late conversion). The influence of SRL on CsA-induced renal injury was evaluated by assessing renal function, histopathology (interstitial inflammation and fibrosis), and apoptotic cell death. Combined CsA and SRL treatment significantly impaired renal function, increased apoptosis, and interstitial fibrosis and inflammation compared with CsA or SRL treatment alone. Early conversion to SRL did not change renal function, histopathology, or apoptosis compared with early CsA withdrawal. By contrast, late conversion to SRL significantly aggravated these parameters compared with late CsA withdrawal. In conclusion, early conversion from CsA to SRL is effective in preventing CsA-induced renal injury in a setting of CsA-induced renal injury.
Keyword
MeSH Terms
Figure
Reference
-
1. Marcén R, Pascual J, Teruel JL, Villafruela JJ, Rivera ME, Mampaso F, Burgos FJ, Ortuño J. Outcome of cadaveric renal transplant patients treated for 10 years with cyclosporine: is chronic allograft nephropathy the major cause of late graft loss? Transplantation. 2001. 72:57–62.2. Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002. 346:580–590.3. Kreis H, Oberbauer R, Campistol JM, Mathew T, Daloze P, Schena FP, Burke JT, Brault Y, Gioud-Paquet M, Scarola JA, Neylan JF. Rapamune Maintenance Regimen Trial. Long-term benefits with sirolimus-based therapy after early cyclosporine withdrawal. J Am Soc Nephrol. 2004. 15:809–817.4. Mota A, Arias M, Taskinen EI, Paavonen T, Brault Y, Legendre C, Claesson K, Castagneto M, Campistol JM, Hutchison B, Burke JT, Yilmaz S, Häyry P, Neylan JF. Rapamune Maintenance Regimen Trial. Sirolimus-based therapy following early cyclosporine withdrawal provides significantly improved renal histology and function at 3 years. Am J Transplant. 2004. 4:953–961.5. Shihab FS, Bennett WM, Yi H, Choi SO, Andoh TF. Sirolimus increases transforming growth factor-1 expression and potentiates chronic cyclosporine nephrotoxicity. Kidney Int. 2004. 65:1262–1271.6. Nielsen FT, Ottosen P, Starklint H, Dieperink H. Kidney function and morphology after short-term combination therapy with cyclosporine A, tacrolimus and sirolimus in the rat. Nephrol Dial Transplant. 2003. 18:491–496.7. Lloberas N, Torras J, Alperovich G, Cruzado JM, Giménez-Bonafé P, Herrero-Fresneda I, Franquesa M, Rama I, Grinyó JM. Different renal toxicity profiles in the association of cyclosporine and tacrolimus with sirolimus in rats. Nephrol Dial Transplant. 2008. 23:3111–3119.8. Kahan BD. The synergistic effects of cyclosporine and sirolimus. Transplantation. 1997. 63:170.9. Schena FP, Pascoe MD, Alberu J, del Carmen Rial M, Oberbauer R, Brennan DC, Campistol JM, Racusen L, Polinsky MS, Goldberg-Alberts R, Li H, Scarola J, Neylan JF. Sirolimus CONVERT Trial Study Group. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation. 2009. 87:233–242.10. Oberbauer R. Protocol conversion from a calcineurin inhibitor based therapy to sirolimus. Transplantation. 2009. 87:S7–S10.11. Kim JY, Lim SW, Li C, Kim JS, Ahn KO, Yang HJ, Choi BS, Kim YS, Kim J, Bang BK, Yang CW. Effect of FTY720 on chronic cyclosporine nephropathy in rats. Transplantation. 2005. 80:1323–1330.12. Song HK, Han DH, Song JH, Ghee JY, Piao SG, Kim SH, Yoon HE, Li C, Kim J, Yang CW. Influence of sirolimus on cyclosporine-induced pancreas islet dysfunction in rats. Am J Transplant. 2009. 9:2024–2033.13. Ghee JY, Han DH, Song HK, Kim WY, Kim SH, Yoon HE, Choi BS, Kim YS, Kim J, Yang CW. The role of macrophage in the pathogenesis of chronic cyclosporine-induced nephropathy. Nephrol Dial Transplant. 2008. 23:4061–4069.14. Shihab FS, Anodoh TF, Tanner AM, Yi H, Bennett WM. Expression of apoptosis regulatory genes in chronic cyclosporine nephrotoxicity favors apoptosis. Kidney Int. 1999. 56:2147–2159.15. Giachelli C, Bae N, Lombardi D, Majesky M, Schwartz S. Molecular cloning and characterization of 2B7, a rat mRNA which distinguishes smooth muscle cell phenotypes in vitro and is identical to osteopontin (secreted phosphoprotein I, 2aR). Biochem Biophys Res Commun. 1991. 177:867–873.16. Li C, Yang CW, Ahn HJ, Kim WY, Park CW, Park JH, Cha JH, Kim J, Kim YS, Bang BK. Colchicine suppresses osteopontin expression and inflammatory cell infiltration in chronic cyclosporine nephrotoxicity. Nephron. 2002. 92:422–430.17. Li C, Lim SW, Sun BK, Choi BS, Glowacka S, Cox A, Kelly D, Kim YS, Kim J, Bang BK, Yang CW. Expression of apoptosis-related factors in chronic cyclosporine nephrotoxicity after cyclosporine withdrawal. Acta Pharmacol Sin. 2004. 25:401–411.18. Han SW, Li C, Ahn KO, Lim SW, Song HG, Jang YS, Cho YM, Jang YM, Ghee JY, Kim JY, Kim SH, Kim J, Kwon OJ, Yang CW. Prolonged endoplasmic reticulum stress induces apoptotic cell death in an experimental model of chronic cyclosporine nephropathy. Am J Nephrol. 2008. 28:707–714.19. Ahn KO, Li C, Lim SW, Song HK, Ghee JY, Kim SH, Kim JY, Yoon HE, Cha JH, Kim J, Yang CW. Infiltration of nestin-expressing cells in interstitial fibrosis in chronic cyclosporine nephropathy. Transplantation. 2008. 86:571–577.20. Yoon HE, Yang CW. Established and newly proposed mechanisms of chronic cyclosporine nephropathy. Korean J Intern Med. 2009. 24:81–92.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Verruca Eradication Following Conversion to Sirolimus in a Renal Transplant Recipient after Longstanding Cyclosporine A Treatment
- A Case of Squamous Cell Carcinoma in Nasal Cavity Treated with Conversion to Sirolimus in a Patient with Kidney Transplantation
- Proliferation Signal Inhibitors: Sirolimus and Everolimus
- Very Late Stent Thrombosis Related to Fracture of a Sirolimus-Eluting Stent
- Post-transplant Proteinuria