J Korean Acad Nurs.
2011 Dec;41(6):834-842. 10.4040/jkan.2011.41.6.834.
Effect of DHEA on Recovery of Muscle Atrophy Induced by Parkinson's Disease
- Affiliations
-
- 1College of Nursing, Seoul National University, Seoul, Korea.
- 2Department of Nursing, Cheongju University, Cheongju, Korea.
- 3Department of Neuropsychiatry, Graduate School of Oriental Medicine, Dongguk University, Gyeongju, Korea.
- 4Dongguk University Research Institute of Biotechnology, Seoul, Korea. jsong0304@dongguk.edu
- KMID: 1120116
- DOI: http://doi.org/10.4040/jkan.2011.41.6.834
Abstract
- PURPOSE
The purpose of this study was to determine the effect of dehydroepiandrosterone (DHEA) on recovery of muscle atrophy induced by Parkinson's disease.
METHODS
The rat model was established by direct injection of 6-hydroxydopamine (6-OHDA, 20 microg) into the left striatum using stereotaxic surgery. Rats were divided into two groups; the Parkinson's disease group with vehicle treatment (Vehicle; n=12) or DHEA treatment group (DHEA; n=22). DHEA or vehicle was administrated intraperitoneally daily at a dose of 0.34 mmol/kg for 21 days. At 22-days after DHEA treatment, soleus, plantaris, and striatum were dissected.
RESULTS
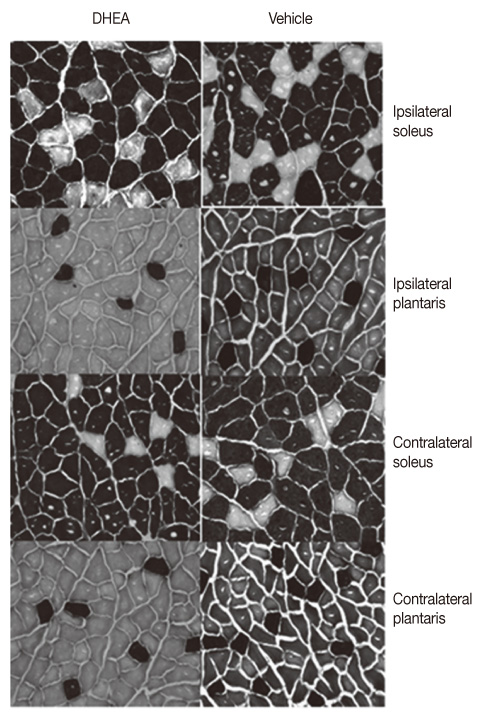
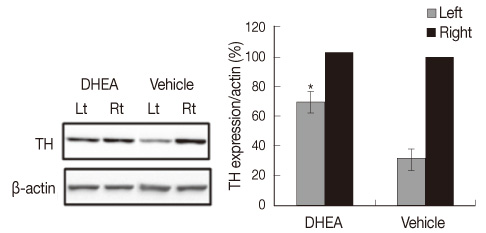
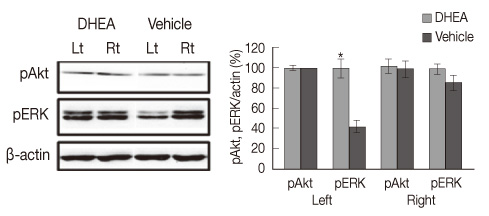
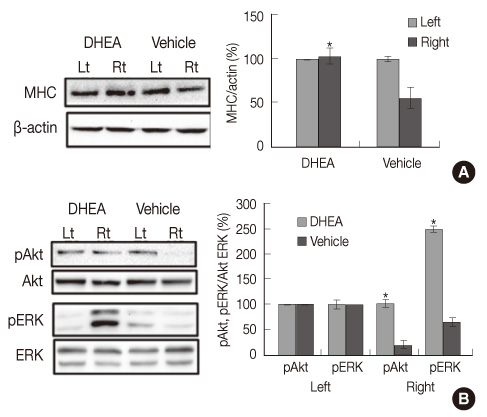
The DHEA group showed significant increase (p<.01) in the number of tyrosine hydroxylase (TH) positive neurons in the lesioned side substantia nigra compared to the vehicle group. Weights and Type I fiber cross-sectional areas of the contralateral soleus of the DHEA group were significantly greater than those of the vehicle group (p=.02, p=.00). Moreover, extracellular signal-regulated kinase (ERK) phosphorylation significantly decreased in the lesioned striatum, but was recovered with DHEA and also in the contralateral soleus muscle, Akt and ERK phosphorylation recovered significantly and the expression level of myosin heavy chain also recovered by DHEA treatment.
CONCLUSION
Our results suggest that DHEA treatment recovers Parkinson's disease induced contralateral soleus muscle atrophy through Akt and ERK phosphorylation.
Keyword
MeSH Terms
-
Animals
Corpus Striatum/drug effects/metabolism
Dehydroepiandrosterone/*pharmacology/therapeutic use
Extracellular Signal-Regulated MAP Kinases/metabolism
Male
Muscle Fibers, Slow-Twitch/drug effects
Muscle, Skeletal/drug effects/metabolism
Muscular Atrophy/drug therapy/*etiology/*pathology
Myosins/metabolism
Neurons/drug effects/enzymology
Oxidopamine/toxicity
Parkinson Disease, Secondary/*chemically induced/*complications
Phosphorylation
Proto-Oncogene Proteins c-akt/metabolism
Rats
Rats, Sprague-Dawley
Tyrosine 3-Monooxygenase/metabolism
Figure
Reference
-
1. American College of Sports Medicine. ACSM's advanced exercise physiology. 2006. Philadelphia: Lippincott Williams & Wilkins.2. Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. The Journal of General Physiology. 1967. 50:197–218.3. Bélanger N, Grégoire L, Bédard P, Di Paolo T. Estradiol and dehydroepiandrosterone potentiate levodopa-induced locomotor activity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine monkeys. Endocrine. 2003. 21:97–101.4. Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: Effects mediated via sigma receptors. Journal of Neuroscience. 1996. 16:1193–1202.5. Bigard XA, Janmot C, Merino D, Lienhard F, Guezennec YC, D'Albis A. Endurance training affects myosin heavy chain phenotype in regenerating fast-twitch muscle. Journal of Applied Physiology. 1996. 81:2658–2665.6. Brown GA, Vukovich MD, Sharp RL, Reifenrath TA, Parsons KA, King DS. Effect of oral DHEA on serum testosterone and adaptations to resistance training in young men. Journal of Applied Physiology. 1999. 87:2274–2283.7. Cano-de-la-Cuerda R, Pérez-de-Heredia M, Miangolarra-Page JC, Muñoz-Hellín E, Fernández-de-Las-Peñas C. Is there muscular weakness in Parkinson's disease? American Journal of Physical Medicine and Rehabilitation. 2010. 89:70–76.8. Choe MA, An GJ. Effect of DHEA administration alone or exercise combined with DHEA before steroid treatment on rat hindlimb muscles. Journal of Korean Academy of Nursing. 2009. 39:321–328.9. Choe MA, An GJ, Lee YK, Im JH, Choi-Kwon S, Heitkemper M. Effect of inactivity and undernutrition after acute ischemic stroke in a rat hindlimb muscle model. Nursing Research. 2004. 53:283–292.10. Cyr M, Calon F, Morissette M, Grandbois M, Di Paolo T, Callier S. Drugs with estrogen-like potency and brain activity: Potential therapeutic application for the CNS. Current Pharmaceutical Design. 2000. 6:1287–1312.11. Frimel TN, Kapadia F, Gaidosh GS, Li Y, Walter GA, Vandenborne K. A model of muscle atrophy using cast immobilization in mice. Muscle and Nerve. 2005. 32:672–674.12. Fryburg DA, Jahn LA, Hill SA, Oliveras DM, Barrett EJ. Insulin and insulin-like growth factor-I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. Journal of Clinical Investigation. 1995. 96:1722–1729.13. Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Progress in Neurobiology. 2001. 63:29–60.14. Harris DS, Wolkowitz OM, Reus VI. Movement disorder, memory, psychiatric symptoms and serum DHEA levels in schizophrenic and schizoaffective patients. World Journal of Biological Psychiatry. 2001. 2:99–102.15. Hornykiewicz O. Ageing and neurotoxins as causative factors in idiopathic Parkinson’s disease-a critical analysis of the neurochemical evidence. Progress in Neuro-psychopharmacology and Biological Psychiatry. 1989. 13(3):19–328.16. Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Molecular and Cellular Biochemistry. 1994. 131:99–104.17. Kim Y, Choe MA. Effect of decreased locomotor activity on hindlimb muscles in a rat model of Parkinson's disease. Journal of Korean Academy of Nursing. 2010. 40:580–588.18. Leskiewicz M, Regulska M, Budziszewska B, Jantas D, Jaworska-Feil L, Basta-Kaim A, et al. Effects of neurosteroids on hydrogen peroxide- and staurosporine-induced damage of human neuroblastoma SH-SY5Y cells. Journal of Neuroscience Research. 2008. 86:1361–1370.19. Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Frontiers in Neuroendocrinology. 2009. 30:65–91.20. Mehta AK, Ticku MK. Unsulfated and sulfated neurosteroids differentially modulate the binding characteristics of various radioligands of GABA(A) receptors following chronic ethanol administration. Neuropharmacology. 2001. 40:668–675.21. Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: Role of estrogen receptor alpha and estrogen receptor beta in neurons and glia. Neuroscience. 2006. 138:851–858.22. Mitoma H, Hayashi R, Yanagisawa N, Tsukagoshi H. Characteristics of parkinsonian and ataxic gaits: A study using surface electromyograms, angular displacements and floor reaction forces. Journal of the Neurological Sciences. 2000. 174:22–39.23. Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SS. The effect of six months treatment with a 100mg daily dose of DHEA on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Journal of Clinical Endocrinology and Metabolism. 1998. 78:1360–1367.24. Morris ME, Iansek R, Matyas TA, Summers JJ. The pathogenesis of gait hypokinesia in Parkinson's disease. Brain. 1994. 117:1169–1181.25. Muir GD, Whishaw IQ. Ground reaction forces in locomoting hemi-parkinsonian rats: A definitive test for impairments and compensations. Experimental Brain Research. 1999. 126:307–314.26. Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Progress in Neurobiology. 1996. 50:275–331.27. Sugino M, Ohsawa N, Ito T, Ishida S, Yamasaki H, Kimura F, Shinoda K. A pilot study of dehydroepiandrosterone sulfate in myotonic dystrophy. Neurology. 1998. 51:586–589.28. Weill-Engerer S, David JP, Sazdovitch V, Liere P, Schumacher M, Delacourte A, et al. In vitro metabolism of dehydroepiandrosterone (DHEA) to 7alpha-hydroxy-DHEA and delta5-androstene-3beta, 17beta-diol in specific regions of the aging brain from Alzheimer's and non-demented patients. Brain Research. 2003. 969:117–125.29. Whishaw IQ, Suchowersky O, Davis L, Sarna J, Metz GA, Pellis SM. Impairment of pronation, supination, and body co-ordination in reach-to-grasp tasks in human Parkinson's disease (PD) reveals homology to deficits in animal models. Behavioural Brain Research. 2002. 133:165–176.30. Yen S, Morales A, Khorram O. Replacement of DHEA in aging men and women: Potential remedial effects. Annals of the New York Academy of Sciences. 1995. 774:128–142.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of DHEA on Hindlimb Muscles in a Focal Cerebral Ischemia Model Rat
- Effect of DHEA Administration Alone or Exercise combined with DHEA before Steroid Treatment on Rat Hindlimb Muscles
- Effects of Treadmill Exercise on the Recovery of Dopaminergic Neuron Loss and Muscle Atrophy in the 6-OHDA Lesioned Parkinson's Disease Rat Model
- Effect of Dehydroepiandrosterone on Affected and Unaffected Hindlimb Muscles in Rats with Neuropathic Pain Induced by Unilateral Peripheral Nerve Injury
- Effect of DHEA Administration before, during and after Dexamethasone Treatment on Body Weight and Mass of TypeI, II Muscles in Rats