Korean J Radiol.
2004 Dec;5(4):231-239. 10.3348/kjr.2004.5.4.231.
Quantitative Evaluation of Liver Function with MRI Using Gd-EOB-DTPA
- Affiliations
-
- 1Department of Radiology, Kyungpook National University School of Medicine, Korea. hkryeom@knu.ac.kr
- KMID: 1118830
- DOI: http://doi.org/10.3348/kjr.2004.5.4.231
Abstract
OBJECTIVE
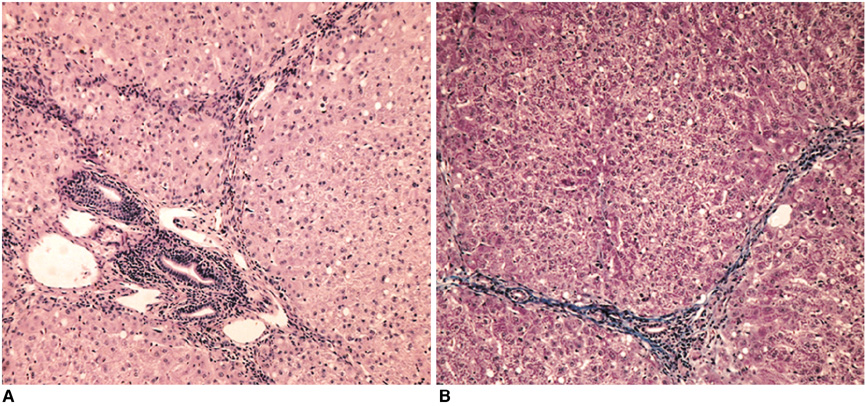
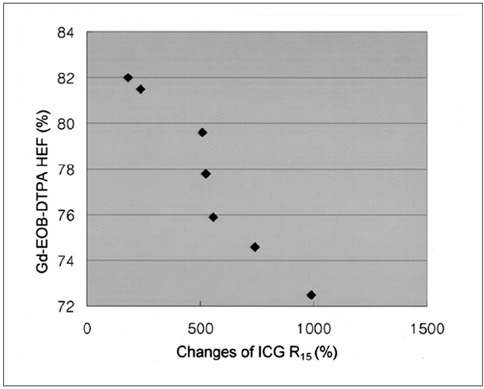
Gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA) is a newly developed MR contrast agent. After intravenous injection, Gd-EOB-DTPA is gradually taken up by the hepatocytes and eventually excreted via the biliary pathway without any change to its chemical structure. Because of these characteristics, it can be used as a tracer for quantitative liver function testing. The purpose of this study is to develop a noninvasive method of quantitation of the hepatic function using Gd-EOB-DTPA through the deconvolution analysis. MATERIALS AND METHODS: Adult New Zealand white rabbits (n = 10, average body weight = 3.5 kg) were used in the present study. Hepatic injury was induced to by the intragastric administration of carbon tetrachloride (CCl4) three times a week for three weeks. Liver enzyme (aspartate aminotransferase, AST; alanine aminotransferase, ALT) levels and the plasma indocyanine green (ICG) retention rate 15 minutes after an intravenous injection of ICG (ICG R15), was checked before and after the three-week administration of CCl4. At the end of experimental period, an observer "blinded" to the treatment given the rabbits performed the histological examination. MRI studies were performed before and after the three-week administration of CCl4 on a 1.5 T scanner using a human extremity coil. After intravenous bolus injection of Gd-EOB-DTPA (0.3 mL of Gd-EOB-DTPA freshly prepared in 2.7 mL of normal saline) through the ear vein, the 250 axial single level dynamic MR images were obtained using a fast low angle shot (FLASH, TR/TE = 11/4.2 msec, flip angle = 15, acquisition time 1 second, slice thickness = 5 mm, matrix = 128x128, field of view = 120 mm) sequence with 1.5 sec time intervals. The time-intensity curves were obtained at the abdominal aorta and the liver parenchyma that was devoid of blood vessels. Deconvolution analysis of the aortic (input function) and hepatic parenchymal (output function) time-intensity curves was performed with a modified Fourier transform technique to calculate the hepatic extraction fraction (HEF). The presence and type of hepatic injury were determined by the histopathologic examination and statistical analysis of the changes of the hepatic enzyme levels, the ICG R15 and Gd-EOB-DTPA HEF values between the time before and after CCl4 administration with Wicoxon signed rank test. Correlation between the Gd-EOB-DTPA HEF and the change of the ICG R15 were analyzed with Pearson's correlation coefficient. RESULTS: Histopathologic examination showed findings that were compatible with hepatic fibrosis caused by chronic liver injury. The initial blood biochemical studies before the administration of carbon tetrachloride showed that the mean AST and ALT levels were 39.8+/-5.2 IU/L and 59.1+/-11.7 IU/L, respectively. The AST and ALT levels increased to 138.4+/-50.5 IU and 172.0+/-71.6 IU/L, respectively, after the three week administration of CCl4. The ALT and AST levels were significantly increased after the three weeks of CCl4 administration (p=0.018). The ICG R15 values were 4.47+/-2.08% and 19.43+/-3.98% before and after three-week administration of CCl4, respectively. The ICG R15 values were significantly increased after hepatic injury (p=0.018). After normalizing the HEF as 100% in each rabbit before CCl4 administration, the deconvoluted curve after CCl4 administration revealed less hepatocyte extraction efficiency with a mean value of 77.7+/-3.6. There was a significant correlation between the HEF and changes of the ICG R15 by the Pearson correlation coefficient assessment (correlation coefficient = -0.965, p=0.000). CONCLUSION: The Gd-EOB-DTPA HEF could be calculated from deconvolution analysis of aortic and hepatic parenchymal time-intensity curves obtained by dynamic MRI. The Gd-EOB-DTPA HEF was well correlated with changes of the ICG R15, which is the most common parameter used in the quantitative estimation of the hepatic function. The Gd-EOB-DTPA HEF is a direct, noninvasive technique for the quantitative evaluation of liver function. It could be a promising alternative for the determination of noninvasive hepatic function in those patients with liver disease.
MeSH Terms
-
Alanine Transaminase/blood/drug effects
Animals
Aspartate Aminotransferases/blood/drug effects
Biological Markers/blood
Carbon Tetrachloride
Coloring Agents/metabolism
Contrast Media/*administration & dosage
Disease Models, Animal
Fibrosis/chemically induced
Gadolinium DTPA/*administration & dosage
Indocyanine Green/metabolism
Injections, Intravenous
Liver/*enzymology/*pathology
Liver Function Tests/*methods
*Magnetic Resonance Imaging
Rabbits
Figure
Reference
-
1. Brensilver HL, Kaplan MM. Significance of elevated liver alkaline phosphatase in serum. Gastroenterology. 1975. 68:1556–1562.2. Cohen JA, Kaplan MM. The SGOT/SGPT ratio: an indicator of alcoholic liver disease. Dig Dis Sci. 1979. 24:835–838.3. Almersjo O, Bengmark S, Hafstrom LO, Olsson R. Enzyme and function changes after extensive liver resection in man. Ann Surg. 1969. 169:111–119.4. Moody FG, Rikker LF, Aldrete JS. Estimation of the functional reserve of human liver. Ann Surg. 1974. 180:592–598.5. Henderson JM, Warren WD. A method of measuring quantitative hepatic function and hemodynamics in cirrhosis: the changes following distal splenorenal shunt. Jpn J Surg. 1986. 16:157–168.6. Hepner GW, Vesell ES. Quantitative assessment of hepatic function by breath analysis after oral administration of [14C] aminopyrine. Ann Intern Med. 1975. 82:632–638.7. Vesell ES. The antipyrine test in clinical pharmacology: conceptions and misconceptions. Clin Pharmacol Ther. 1979. 26:275–286.8. Hunton DB, Bollman JL, Hoffman HN. Studies of hepatic functions with indocyanine green. Gastroenterology. 1960. 39:713–723.9. Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993. 9:298–304.10. Hashimoto M, Watanabe G. Simultaneous measurement of effective hepatic blood flow and systemic circulation. Hepatogastroenterology. 2000. 47:1669–1674.11. Berk PD, Potter BJ, Stremmel W. Role of plasma membrane ligand binding proteins in the hepatocellular uptake of albumin-bound organic anions. Hepatology. 1987. 7:165–176.12. Cherrick GR, Stein SW, Leevy CM, Davidson CS. Indocyanine green: observation on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960. 39:592–600.13. Wolkoff AW. The role of an albumin receptor in hepatic organic anion uptake: the controversy continues. Hepatology. 1987. 7:777–779.14. Hashimoto M, Watanabe G. Hepatic parenchymal cell volume and the indocyanine green tolerance test. J Surg Res. 2000. 92:222–227.15. Murase K, Tsuda T, Mochizuki T, Ikezoe J. Hepatic excretion fraction of hepatobiliary radiopharmaceuticals measured using spectral analysis. Nucl Med Commun. 1999. 12:1041–1045.16. Tolia V, Kottamasu SR, Tabassum D, Simpson P. The use of hepatocyte extraction fraction to evaluate neonatal cholestasis. Clin Nucl Med. 1999. 24:655–659.17. Brensilver HL, Kaplan MM. Significance of elevated liver alkaline phosphatase in serum. Gastroenterology. 1975. 68:1556–1562.18. Proctor E, Chatamra K. Controlled induction of cirrhosis in the rat. Br J Exp Pathol. 1983. 64:320–330.19. Bosma A, Brouwer A, Seifert WF, Knock DL. Synergism between ethanol and carbon tetrachlorid in the generation of liver fibrosis. J Pathol. 1988. 156:15–21.20. McLean EK, McLean AE, Sutton PM. Instant cirrhosis: an improved method for producing cirrhosis of the liver in rats by simultaneous administration of carbon tetrachloride and phenobarbitone. Br J Exp Pathol. 1969. 50:502–506.21. Brandao CG, Ferreira HH, Piovesana H, et al. Development of an experimental model of liver cirrhosis in rabbits. Clin Exp Pharmacol Physiol. 2000. 27:987–990.22. Schuhmann-Giampieri G, Mahler M, Roll G, Maibauer R, Schmitz S. Pharmacokinetics of the liver-specific contrast agent Gd-EOB-DTPA in relation to contrast-enhanced liver imaging in humans. J Clin Pharmacol. 1997. 37:587–596.23. Muhler A, Oude Elferink RPJ, Weinmann HJ. Complete elimination of the hepatobiliary MR contrast agent in hepatic dysfunction: an experimental study using transport-deficient, mutant rats. MAGMA. 1994. 1:134–139.24. Clement O, Muhler A, Vexler V, Berthezene Y, Brasch RC. Gadolinium-ethoxybenzly-DTPA: a new liver-specific magnetic resonance contrast agent. Kinetic and enhancement patterns in normal and cholestatic rats. Invest Radiol. 1992. 27:612–619.25. Muhler A, Clement O, Saeed M, et al. Gadolinium-ethoxybenzyl-DTPA, a new liver-directed magnetic resonance contrast agent. Absence of acute hepatotoxic, cardiovascular, or immunogenic effects. Invest Radiol. 1993. 28:26–32.26. Weinmann HJ, Schuhmann-Giampieri G, Schmitt-Willich H, Vogler H, Frenzel T, Gries H. A new lipophilic gadolinium chelate as a tissue-specific contrast medium for MRI. Magn Reson Med. 1991. 22:233–237.27. Muhler A, Heinzelmann I, Weinmann HJ. Elimination of gadolinium-ethoxybenzyl-DTPA in a rat model of severely impaired liver and kidney excretory function. An experimental study in rats. Invest Radiol. 1994. 29:213–216.28. Hamm B, Staks T, Muhler A, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995. 195:785–792.29. Muhler A, Freise CE, Kuwatsuru R, et al. Acute liver rejection: evaluation with cell-directed MR contrast agents in a rat transplantation model. Radiology. 1993. 186:139–146.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Supradiaphragmatic Liver Confirmed by a Hepatocyte-specific Contrast Agent (Gd-EOB-DTPA): A Case Report
- Quantitative Evaluation of the Hepatic Parenchymal Change in Patients with Chronic Liver Disease Using Gd-EOB-DTPA-enhanced MRI: Comparison with Normal Liver
- Clinical usefulness of T1-weighted MR cholangiography with Gd-EOB-DTPA for the evaluation of biliary complication after liver transplantation
- Enhancement Pattern of Liver Parenchyma during Late Dynamic Phase Imaging: Comparison between Gd-EOB-DTPA and Gd-DTPA-BMA
- Hepatic Lymphoma Representing Iso-Signal Intensity on Hepatobiliary Phase, in Gd-EOB-DTPA-Enhanced MRI: Case Report